
allied
academies
Page 34
Journal of Industrial and Environmental Chemistry
|
Volume 2
GREEN CHEMISTRY &
TECHNOLOGY
7
th
International Conference on
J u n e 1 8 - 2 0 , 2 0 1 8 | D u b l i n , I r e l a n d
Jakob Köchermann et al., J Ind Environ Chem 2018, Volume 2 | DOI: 10.4066/2591-7331-C1-002
PRODUCTION OF FURFURAL FROM
D-XYLOSE AND ORGANOSOLV
HEMICELLULOSE IN WATER/ETHANOL
MIXTURES
Jakob Köchermann, J Schreiber
and
M Klemm
DBFZ German Biomass Research Centre gGmbH, Germany
I
n recent years the production of furfural fromD-xylose and hemicellulose
richstreams fromthepulpandpaper industrywereextensivelydiscussed.
However, one problem that has always been described was the formation
of insoluble humins by self- and cross-polymerization of furfural. Due
to these side reactions the product yield and selectivity decreases.
Therefore, the use of biphasic systems or ionic liquids was investigated
to avoid this issue. Promising results have been shown but such systems
could by costly due to expensive solvents and the subsequently recovery
processes. Another approach to suppress polymerization of furfural is
the usage of alcohol/water mixtures as reaction medium. The alcohol
can react with the sugars and stabilize the reactive intermediates. For
our exploration as reaction medium ethanol/water with different mass
ratios were tested. Xylose was used as model compound for organosolv
hemicellulose and sulphuric acid as homogeneous catalyst. The
experiments were conducted in a thermostatically heated 500 mL stirred
batch reactor at three temperatures (180, 200 and 220°C). To avoid the
heating phase, the xylose was dissolved in water and transferred in a
liquid charging pipette made of stainless steel. The educt solution was
added to the ethanol/water mixture only after reaching the reaction
temperature. Immediately after addition, a first sample was taken by a
liquid sample valve with dip tube. Five more samples followed after 5, 15,
30, 60 and 180 min. Afterwards the reactor was cooled down to ambient
by the thermostat as fast as possible. Subsequently the insoluble humins
were separated from the reaction medium by vacuum filtration. The
process liquor samples were analysed by liquid chromatography (HPLC-
DAD) and the humins were quantified by weighing. Since ethanol is used
as solvent for the organosolv process, the hemicellulose stream after the
digestion contains residues of that alcohol. Therefore, we were interested
on furfural polymerization suppress capacity of different ethanol/water
mixtures. This approach has an interesting benefit since after furfural
separation ethanol/water stream can be reused for the organosolv
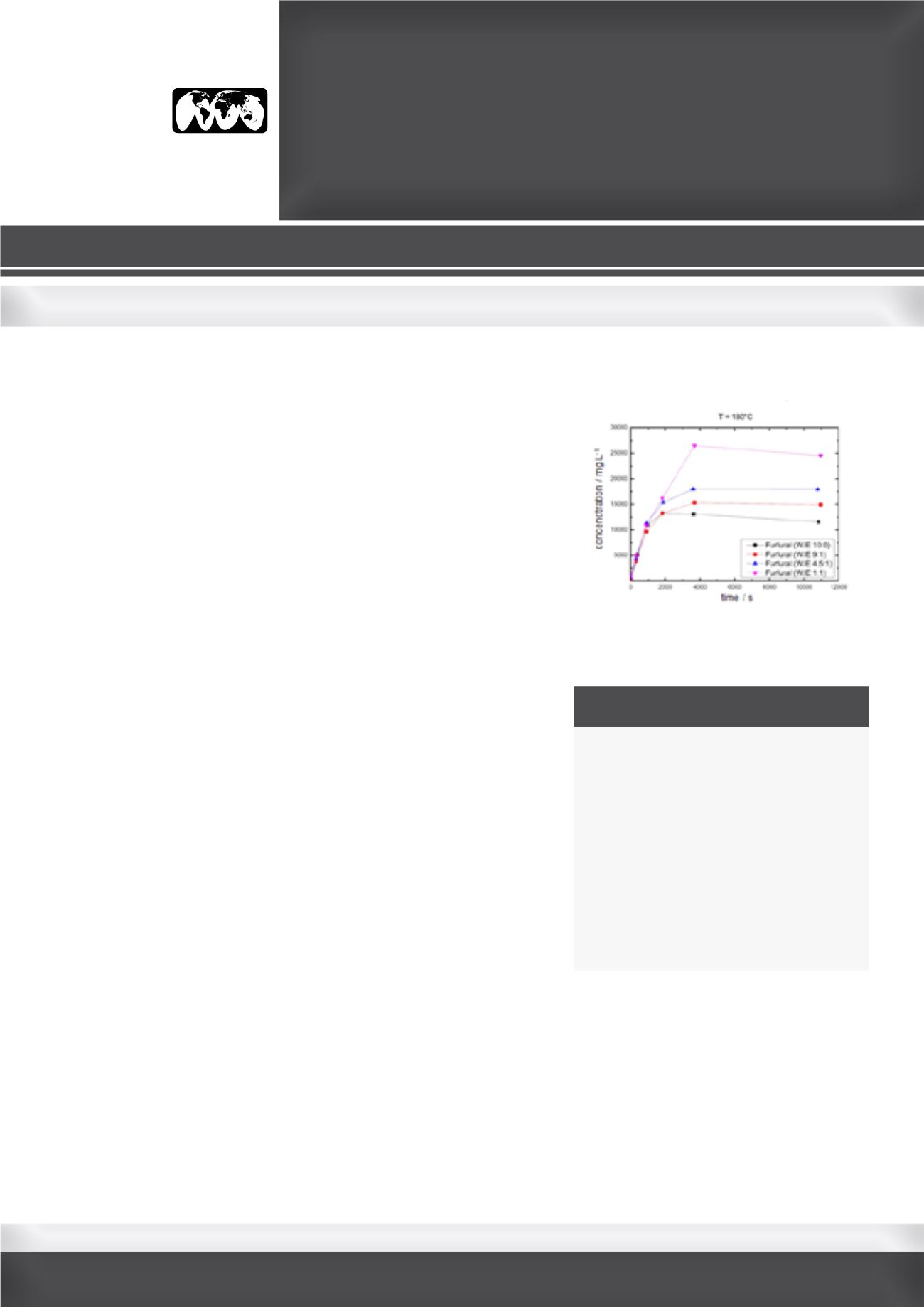
process. Preliminary results show a correlation between the ethanol/
water ratio and the amount of formed humins. The ethanol content has
also a strong influence and leads to an increase of furfural yield (Fig.1).
Furthermore, reference experiments with pure water were conducted. The
Jakob Köchermann studied chemical engineer-
ing at the Karlsruhe Institute of Technology (KIT)
and Technical University of Dresden (TUD). He
received his diploma (equiv. to MSc) in 2014 at
TUD. In 2015 he joined German Biomass Re-
search Center, where he worked until 2016 as
research fellow, focusing on hydrothermal con-
version of lignocellulosic biomass. Since 2016
he is PhD fellow at German Biomass Research
Center. Within the framework of his PhD thesis,
Jakob Köchermann works on hydrothermal
conversion of organosolv hemicellulose and xy-
lose to furfural.
jakob.kö chermann@dbfz.deBIOGRAPHY
results are promise for further explorations
with real organosolv hemicellulose hat should
be carried out in the next step.
Figure: Furfural concentration at various
water/ethanol mass ratios and a reaction
temperature of 180°C
















