
allied
academies
Page 50
Journal of Industrial and Environmental Chemistry
|
Volume 2
GREEN CHEMISTRY &
TECHNOLOGY
7
th
International Conference on
J u n e 1 8 - 2 0 , 2 0 1 8 | D u b l i n , I r e l a n d
Filipa B Pimentel et al., J Ind Environ Chem 2018, Volume 2 | DOI: 10.4066/2591-7331-C1-002
TOTAL PHENOLICS OF
GRACILARIA
VERMICULOPHYLLA
: OPTIMIZING
EXTRACTION PROCEDURES USING
GREEN METHODOLOGIES
Filipa B Pimentel, Rita C Alve
and
M Beatriz P P Oliveira
University of Porto, Portugal
S
eaweeds are an important source of natural compounds with
recognized health benefits. One of the world’s most cultivated and
valuable seaweed is
Gracilaria
. This species is mainly cultivated and
harvested to extract phycocolloids, providingmore than 50% of the world’s
supply of the agar used by the food and cosmetic industries. The interest
in this species goes beyond this, taking into account its composition in
secondary metabolites with biological activity, which include phenolics.
Overall, these compounds are presumed to protect algal thalli from UV
radiation and to act as free-radical scavenging agents. This study aimed
to optimize an extraction protocol, using green chemistry principles, for
further quantificationof the total phenolics of
G. vermiculophylla
. The dried
samples, produced in an Integrated Multi-Trophic Aquaculture (IMTA)
system (Aveiro, Portugal), were thoroughly ground and homogenized prior
to analysis. Samples were submitted to several extraction conditions in
which the ratio of sample:solvent:time of extraction were tested, varying
one parameter at a time. The extraction procedure was optimized using
water as the extraction solvent in the following ratios: 1:30, 1:40 and 1:50
(w:v), during 30, 60, 90 up to 120 minutes, at room temperature. Some of
the protocols comprised re-extractions of the samples every 30 minutes,
while in others the extraction was continuous for the stipulated period of
time. Once the optimum extraction conditions were reached, the same
protocol was applied to the samples, using two other solvents: an hydro-
ethanolic solution (1:1, v:v) and ethanol. Results of the optimization
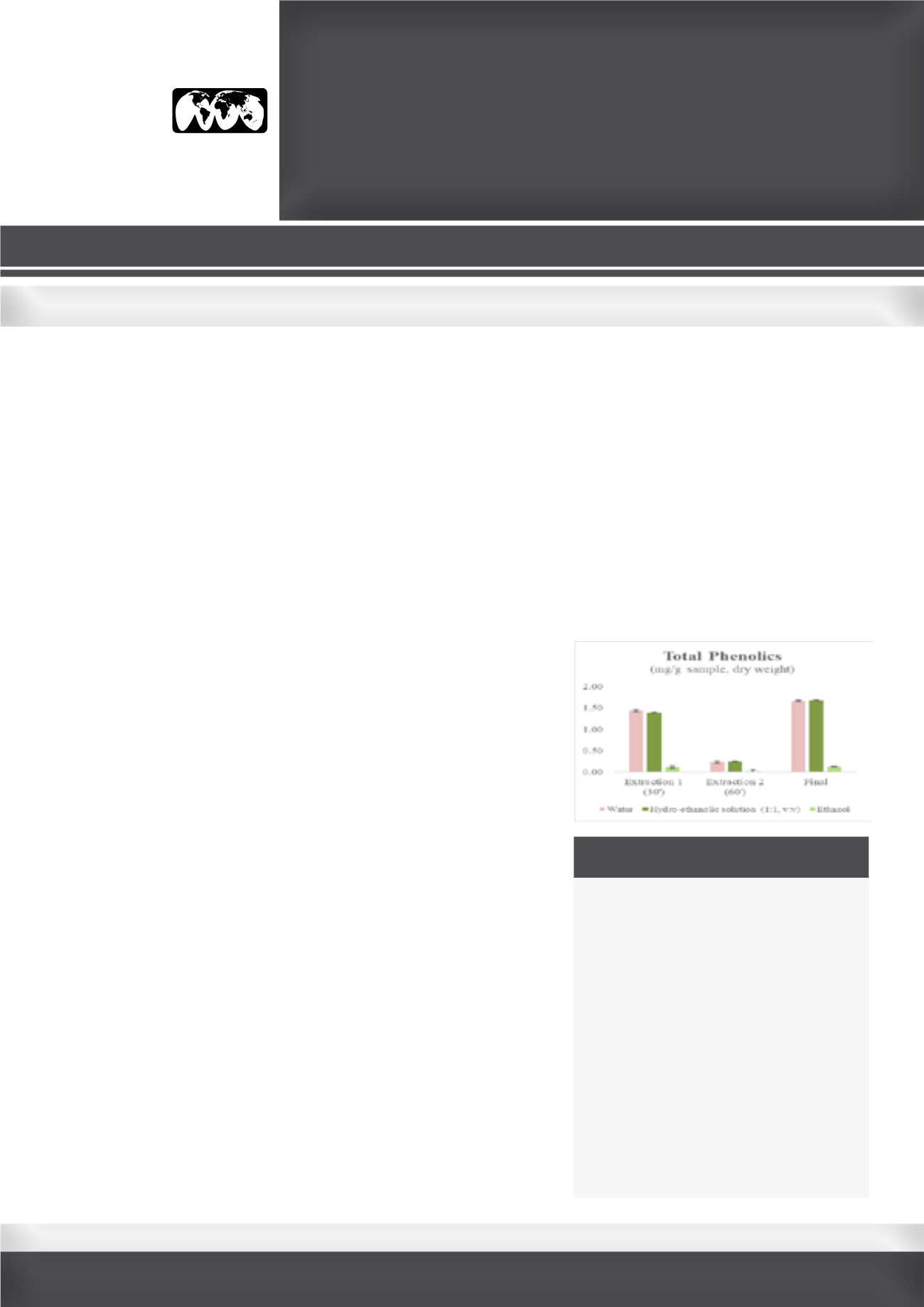
protocols using water as the extraction solvent show that the total
phenolics (TP) varied between 0.88±0.05 and 1.66±0.03 mg/g sample
(dry weight). The higher amounts of TP were obtained at a ratio of 1:40
(w:v) for 30 minutes with an additional 30 minutes re-extraction with ¼
of the total volume. Continuous extractions did not present advantages
over protocols comprising re-extractions over the time. In fact, in most
cases, a slight decrease of the TP is observed over time, probably due to
the degradation of the compounds. Comparing the water and the hydro-
ethanolic solution, in the first extraction, water was more efficient, but, at
the end of the process, there were no significant differences between the
amounts of TP in both solvents (1.66±0.03 and 1.68±0.03 mg/g sample
dw, respectively; p<0.05). Ethanol was the least efficient solvent to
extract TP (0.12±0.0 mg/g sample dw). The present work was designed
Filipa B Pimentel is a PhD student in Pharma-
ceutical Sciences (Nutrition and Food Science
Speciality) at the Faculty of Pharmacy of the
University of Porto, Portugal. Since 2012, she
is a researcher of REQUIMTE (Rede de Quími-
ca e Tecnologia), the largest network in Chem-
istry and Chemical Engineering established in
Portugal, which is focused on the development
of Sustainable Chemistry. Her research activi-
ties have been developed at the Department of
Chemical Sciences of the Faculty of Pharmacy
of the University of Porto in the food chemistry
and nutrition fields. She has 21 publications,
cited over 150 times. Formerly, she completed
her degree in Nutrition Sciences at the Faculty
of Nutrition and Food Sciences of the University
of Porto in 2004, and a Master in Food Service
Management from the same Faculty in 2011.
filipabpimentel@gmail.comBIOGRAPHY
to investigate the optimum extraction
protocol of the TP of
G. vermiculophylla
, using
green chemistry principles. The optimum
conditions were selected after combining
the following parameters: the recovery of
the maximum amount of TP, using the eco-
friendliest solvent at the lesser amounts
possible, during the shortest period of time.
In this case, this conducted us to select water
as the extractor solvent, using the ratio of
1:40 (w:v) for 30 minutes with an additional
30 minutes re-extraction with ¼ of the total
volume. This process allows the recovery of
natural seaweed-derived antioxidants, which
can be safely used for food and cosmetics
applications.














