
allied
academies
Journal of Biotechnology and Phytochemistry
Volume 1 Issue 3
Chemistry World 2017
Notes:
Page 44
November 13-15, 2017 Athens, Greece
7
th
World Congress on
Chemistry
Synthesis and properties of two classes of thiazole-
based organoboron fluorophores possessing the AIEE/
AIE effect
Belskaya Nataliya
1
, Kseniya I Lugovik
1
, Alexander K Eltyshev
1
, Polina O
Suntsova
1
, Pavel Slepukhin
2
and
Enrico Benassi
3
1
Ural Federal University, Russia
2
Ural Branch of Russian Academy of Science, Russia
3
Nazarbayev University, Republic of Kazakhstan
L
uminescent organoboron molecular systems have been
receiving an increasing amount of interest over the past
few decades. The presence of boron in the structure of organic
compounds leads to substantialmodifications in themolecule’s
electronic structure due to the electron deficiency of boron.
Some boron complexes featuring diketones, iminoketones
azo dyes, β-enaminones, β-ketoiminates, and hydrazones
have shown high fluorescence in the solid state and either
aggregation-induced emission (AIE) or aggregation-induced
emission enhancement (AIEE). However, although BF2
complexes are actively being synthesized and investigated, only
a few examples of AIE/AIEE-active luminophores have been
reported in the literature due to the deficiency of appropriate
frameworks. Moreover, the number of known AIE/AIEE-
active boron complexes that efficiently emit in the solid state
is still limited. We recently reported the synthesis and photo
physical characteristics of the aryl enamine and aryl aza-
enamine thiazoles, which show weak fluorescence in solution
that was proven to be caused by intramolecular hydrogen
bonding and that is very sensitive to the microenvironment.
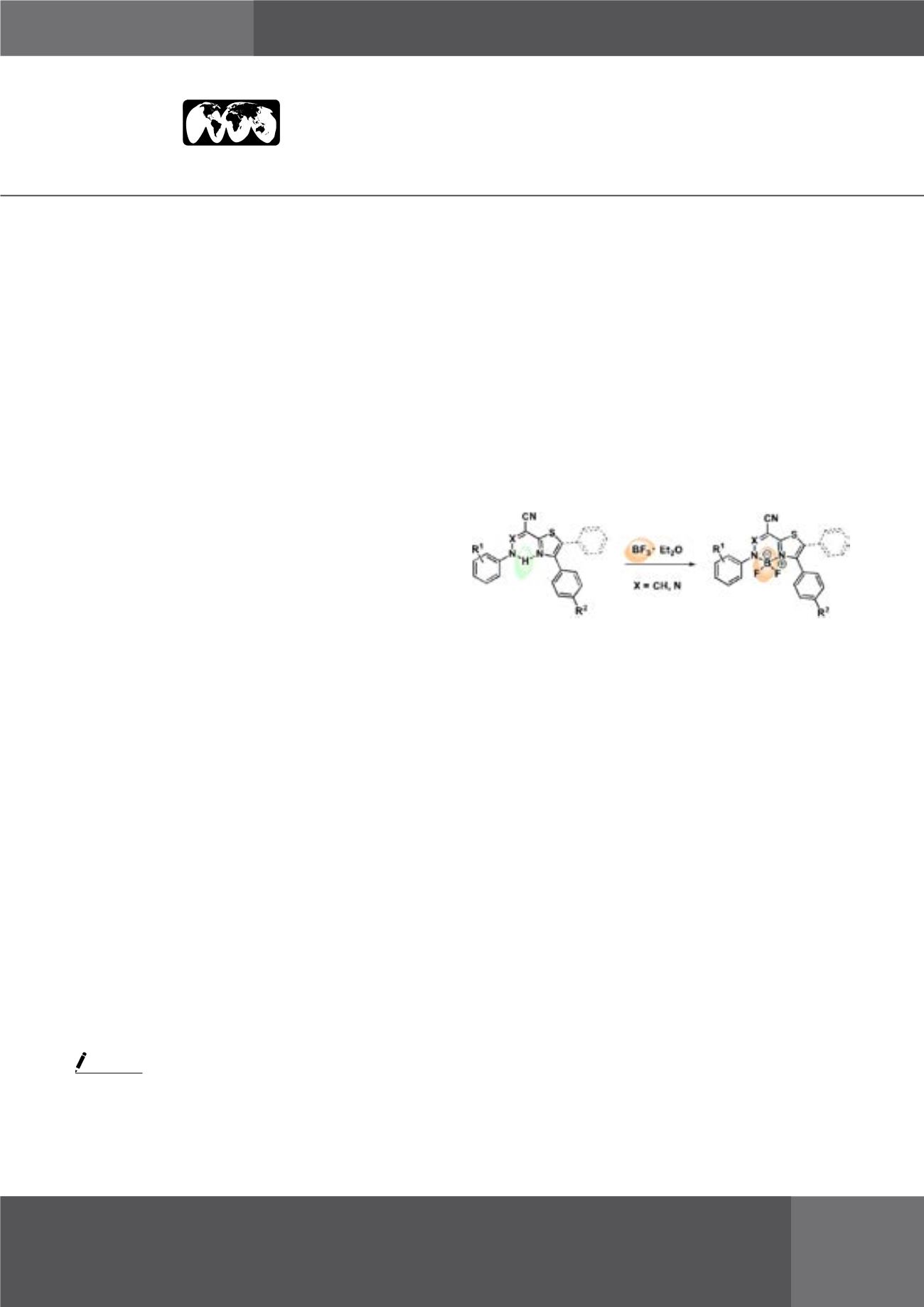
The structure of the these thiazole derivatives includes a
convenient combination of functionalities and may be used
as a bidentante N, N-ligand to synthesize BF2 complexes. We
report now about synthesis of two new series of promising
thiazole-containing BF2 complexes and their spectroscopic
and photo physical characterization. These investigations
were performed in solution, powder and aggregation states.
Solvato(fluoro)chromism was also explored. Some structure-
property relationships were rationalized by DFT calculations.
Biography
Belskaya Nataliya has completed her Doctor Sciences thesis at Ural Federal
University, Russia in 2011. She was appointed as Associate Professor at Ural
Federal University in 2006, becoming a full Professor at the same university
in 2012. Till 2017, 75 publications have appeared in international and Russian
journals about her work that have been cited over 200 times. Her research is
focused on the chemistry of heterocyclic compounds, pericyclic reaction and
design, synthesis and investigation of fluorescent compounds in the solutions,
suspension and solid state.
n.p.belskaya@urfu.ruBelskaya Nataliya et al., J Biotech and Phyto 2017
















