
allied
academies
Journal of Biotechnology and Phytochemistry
Volume 1 Issue 3
Chemistry World 2017
Notes:
Page 43
November 13-15, 2017 Athens, Greece
7
th
World Congress on
Chemistry
Facile synthesis, fluorescence and functional properties
of π-expanded fluorenes
Jagarapu Ramakrishna
and
Parthasarathy Venkatakrishnan
Indian Institute of Technology Madras, India
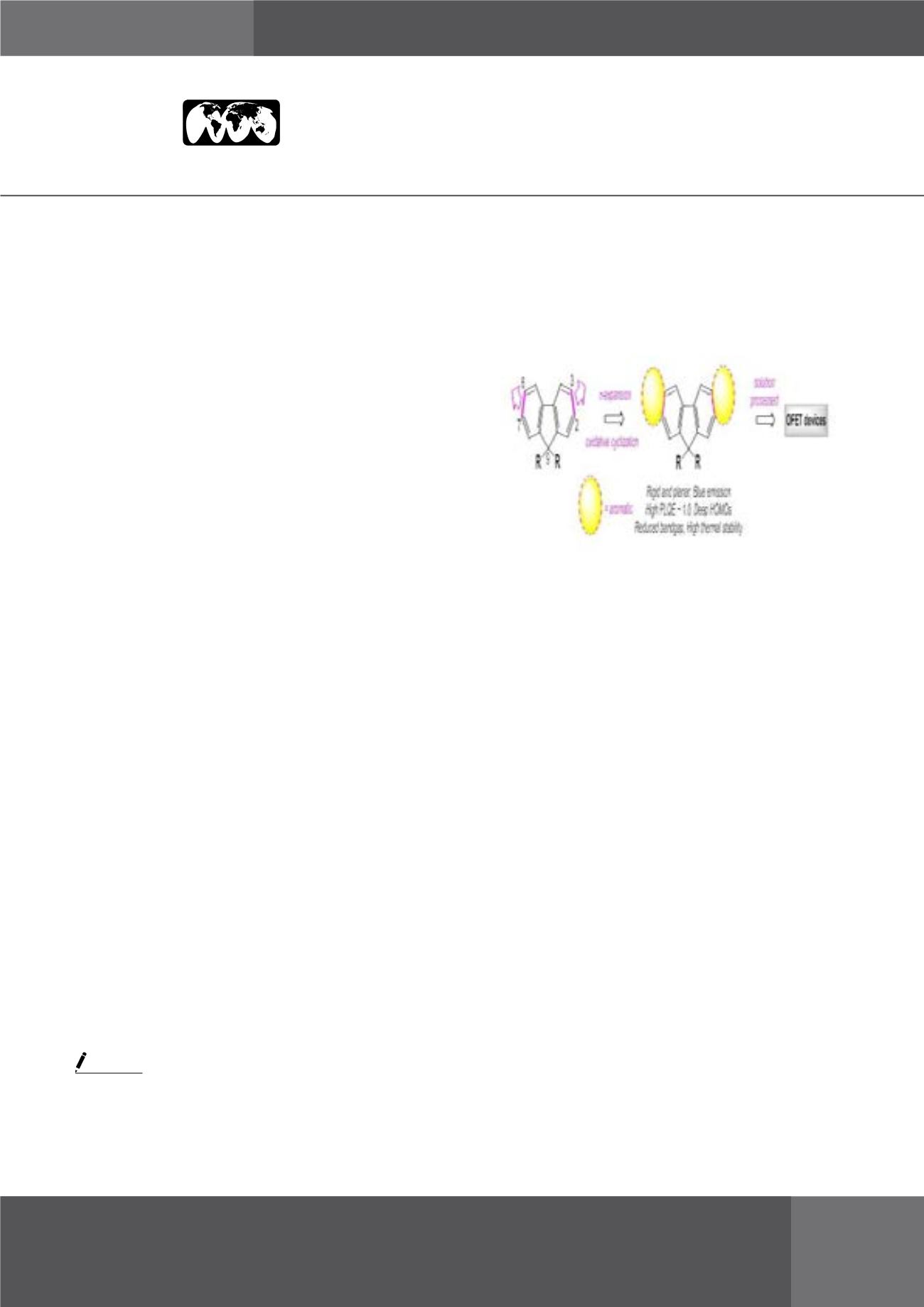
π
-Expanded 2D fluorenes were synthesized via double
annulation at 2,3 and 6,7-faces of fluorene employing
DDQ-mediated oxidative cyclization method with very high
regioselectivity. All the newly synthesized larger fluorenes
were thoroughly characterized by 1H and 13C NMR, IR
spectroscopy, and high-resolution mass spectrometry. The
rigid and planar fluorenes thus obtained led to near-UV
absorbance, bright blue emission with very high close-to-
unity fluorescence quantum yields, and deep HOMO energy
levels with excellent thermal stabilities. In addition, single
crystal X-ray analyses of the newly synthesized fluorenes
revealed potential π-π stacking that was found to depend on
the substituents at either 9-position or at the aromatic ring. As
these electron-rich fluorenes are very well soluble in common
organic solvents, we have fabricated OFET devices for them
via the solution-process method and have characterized their
charge transport performances. Some of these interesting
results will be showcased in this presentation.
Biography
Jagarapu Ramakrishna is currently pursuing his PhD in Functional Organic
Materials under the guidance of P Venkatakrishnan at IIT Madras, India. He is
currently in final year of his PhD degree and expecting to defend his thesis early
in 2018. His research interests are in the area of Synthetic Organic Chemistry,
Organic Photonic, Electronic and Energy Materials.
ramakrishna87.j@gmail.comJagarapu Ramakrishna et al., J Biotech and Phyto 2017
















