
Page 21
allied
academies
J u n e 2 8 - 2 9 , 2 0 1 8 | A m s t e r d a m , N e t h e r l a n d s
Joint Event on
OBESITY AND WEIGHT MANAGEMENT
VACCINES AND IMMUNOLOGY
&
International Conference on
International Conference on
Asian Journal of Biomedical and Pharmaceutical Sciences
|
Volume 8
ISSN:
2249-622X
CHARACTERISTICS OF MONTANIDE™
ISA 51 VG ADJUVANT DESIGNED FOR
THERAPEUTIC CANCER VACCINES
Maria Lazaro
Seppic, France
T
herapeutic cancer vaccines are one interesting alternative to treat cancer
by active immunotherapy. The use of well-defined overexpressed tumor
antigens is linked with weak and short term immune response. To improve
the immune response induced antigens may be associated with enhancers
such as adjuvants. Water-in-oil (W/O) emulsions such as MontanideTM ISA
51 VG represent an interesting option for immunotherapy vaccines for which
potent adjuvants are required. CIMAVAX-EGF vaccine to treat cancer has
already been authorized in Cuba and many others latin american countries,
it’s also in late state in Europe and Asian countries which efficacy has been
largely proven in patients suffering from lung cancer (NSCLC). Vaccines
based on Montanide™ ISA 51 VG interestingly enhance the immune response
thanks to a depot effect conferred by this kind of adjuvant at the injection
site. This renders a danger signal that increases and prolongs the interaction
with antigen presenting cells. These interactions lead to an enhanced CD8+
and CD4+ activation and promote production of IFN, TNFα, IL-2. Additionally,
the use of adjuvant enhances the memory T-cells, in particular the central
memory T-cells. Taken together, these results show that vaccines based on
Montanide™ ISA 51 VG can induce a potent specific cytotoxic T response and
a significant increase in antibody titers with the development of polarized Th1
responses.
Maria Lazaro is a Pharmacist from Com-
plutense University of Madrid. He/She(accord-
ing to the author’s gender) holds advanced
master’s in Biotechnology and Pharmaceutical
Management works for Seppic in Human Bio-
logicals department (adjuvants for vaccines
and excipients for injectables ) since 2012.
maria.lazaro@airliquide.comBIOGRAPHY
Maria Lazaro, Asian J Biomed Pharmaceut Sci 2018, Volume 8 | DOI: 10.4066/2249-622X-C1-002
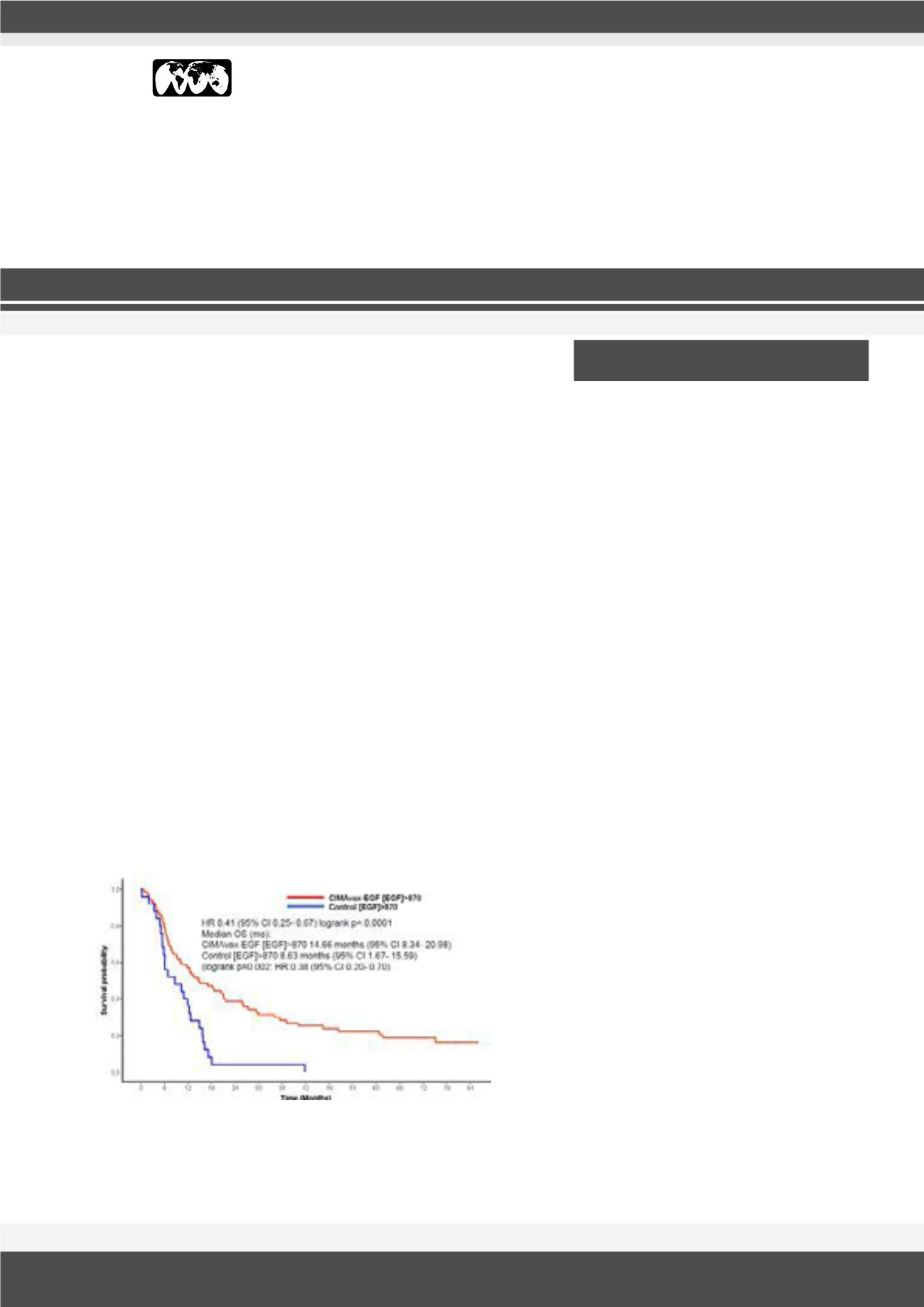
Fig.1:
Kaplan–Meier curve in patients with high [EGF] at day 0. MST for
vaccinated patients was 14.66 months (95% CI, 8.34–20.98) versus 8.63
months (95% CI, 1.67–15.59) for controls.
















