
J u n e 1 1 - 1 3 , 2 0 1 8 | D u b l i n , I r e l a n d
allied
academies
Page 56
CANCER STEM CELLS AND
ONCOLOGY RESEARCH
11
th
International Conference on
Journal of Medical Oncology and Therapeutics
|
Volume 3
Barbara Bessette et al., J Med Oncl Ther 2018, Volume 3
NEUROTROPHINS RECEPTORS: NEW
AGGRESSIVENESS MARKERS IN
GLIOBLASTOMA?
Barbara Bessette
1
, G Bégaud
1
, S Saada
1
, N Vedrenne
1
, E
Deluche
1,2
, S Pinet
1
, S Jawhari
1
, A Boukredine
1
, K Durand
1,2
,
S Robert
1,2
, M O Jauberteau
1
, S Battu
1
, M Verdier
1
and
F
Lalloué
1
1
Université de Limoges, France
2
CHU Limoges, France
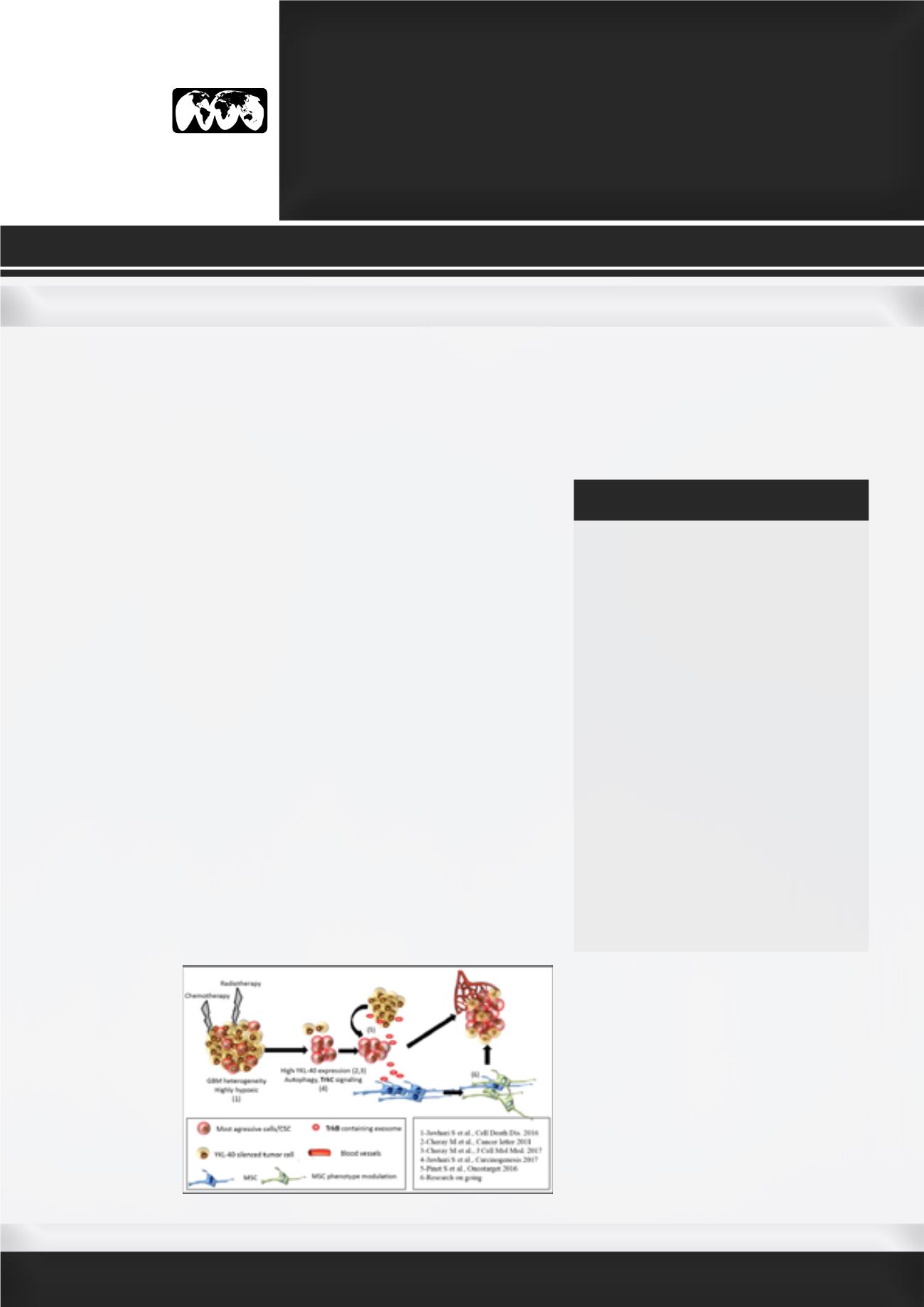
G
lioblastoma (GBM) is the worst brain tumor with therapeutic
resistance and recurrence due to its strong cell heterogeneity, which
relies on cancer stem-like cells’ presence. Tumor aggressiveness is
associated to cancer cell adaptation to their environment: autophagy
process enhancement, the increase of growth factors signaling such as
neurotrophins (TrkB/BNNF and TrkC/NT3), microenvironment modulation
by mesenchymal stem cells (MSC). The high level of hypoxia commonly
encountered in GBM is counterbalanced by the tumor autophagic
capability and growth factors signaling activation. We demonstrated that
an increase of autophagy precedes TrkC/NT3 pathway activation in GBM
cells. Enhancement of both TrkC and NT-3 followed by the increase of
p38MAPK phosphorylation, suggesting the occurrence of a survival loop
that was also underlined in patient’s tumors. However, the double inhibition
of autophagy and TrkC signaling was the only one able to bring cells
apoptosis. The ability of cancer cells, to shape tumor environment through
exosomes release could explain the spreading of “therapeutic resistance”
to neighboring cells. The “stemness” properties loss showed in YKL-40-
silenced cells can be reversed by TrkB-containing exosomes provide by
native cells. This process contributes to restore cell proliferation and to
promote endothelial cell activation. In a xenograft model, TrkB-depleted
e x o s o m e s
from YKL-40-
silenced cells
inhibits tumor
growth
in vivo
.
Our
recent
works showed
changes
in
MSC behavior
Barbara Bessette received her PhD degree in
Neuroscience and oncology from the Universi-
ty of Limoges, France in 2006. She worked one
year in Paris on pediatric brain tumors and the
characterization of cancer stem cells in these
tumors. She followed her post-doctoral experi-
ence by collaborating and working for 3 years
on GLIADYS project with IDD-Biotech (Interna-
tional Drug Development Biotech), specialized
in monoclonal antibodies production in Lyon,
France. The project consisted to develop new
therapeutics for gliomas. During this project,
she develops partner relationship with Oncome-
dics (CRO specialized in Individualized tumor
response tests). She is currently a full-time as-
sistant Professor at the University of Limoges
in the Department of Physiology and she leads
research into HCP-CAPTur team. Her current
research activity focuses on cancer stem cells
in glioblastoma and the role of neuropeptides in
their therapeutic resistance capacity. One of the
workpackages leaders in SUMCASTEC (H2020
European Project) she participates to determine
cancer stem cell electromagnetic signature in
glioblastoma and medulloblastoma.
barbara.bessette@unilim.frBIOGRAPHY
“aggressiveness” by the GBM cell “secretion”,
following irradiations suggesting a putative
link with neurotrophin receptor. Our data
suggest that neurotrophin and their receptors
could be considered as new relevant diagnosis
biomarkers and potential therapeutic targets
in glioblastoma.
Figure: GBM aggressiveness modelization
















