
Page 41
Notes:
allied
academies
February 28-March 01, 2019 | Paris, France
Palliative Care, Obstetrics and Gynecology
Stroke and Clinical Trials
International Conference on
Joint Event on
International Conference on
&
Journal of Research and Reports in Gynecology and Obstetrics | Volume: 3
Structure-function properites of
Kytococcus sedentarius
WhiB1
Meshari A Alhadlaq
University of Sheffield, UK
K
ytococcus sedentarius (Ks) is an opportunistic bacterium
involved in pitted keratolysis, cerebral cyst infections,
endocarditis and bacteraemia. WhiB-like (Wbl) proteins are
a family of proteins that are only located in actinomycetes
and play important role in developmental processes. The
C-terminal regoins are rich in positively charged amino acids,
suggesting a role in DNA-binding. The N-terminal regions
possess four conserved cysteine residues that act as anchors
for iron-sulfur clusters, which respond to redox stress. This
study shows that: (i) the cluster can be isolated in three
forms. (ii) The cluster is important to structure the protein.
(iii) The cluster is sensitive to spermine nitric oxide (NO)
but not oxygen (O
2
). Significence: the iron-sulfure cluster
of WhiB1 is a key factor in the protein function. The cluster
modulates the conformation of the protein, changes the
DNA-binding properties and allows the protein to respond
to NO but not O
2
. These facts suggest that WhiB1 has a role
as an NO-responsive gene regulator that could be important
for survival and persistence in human macrophages.
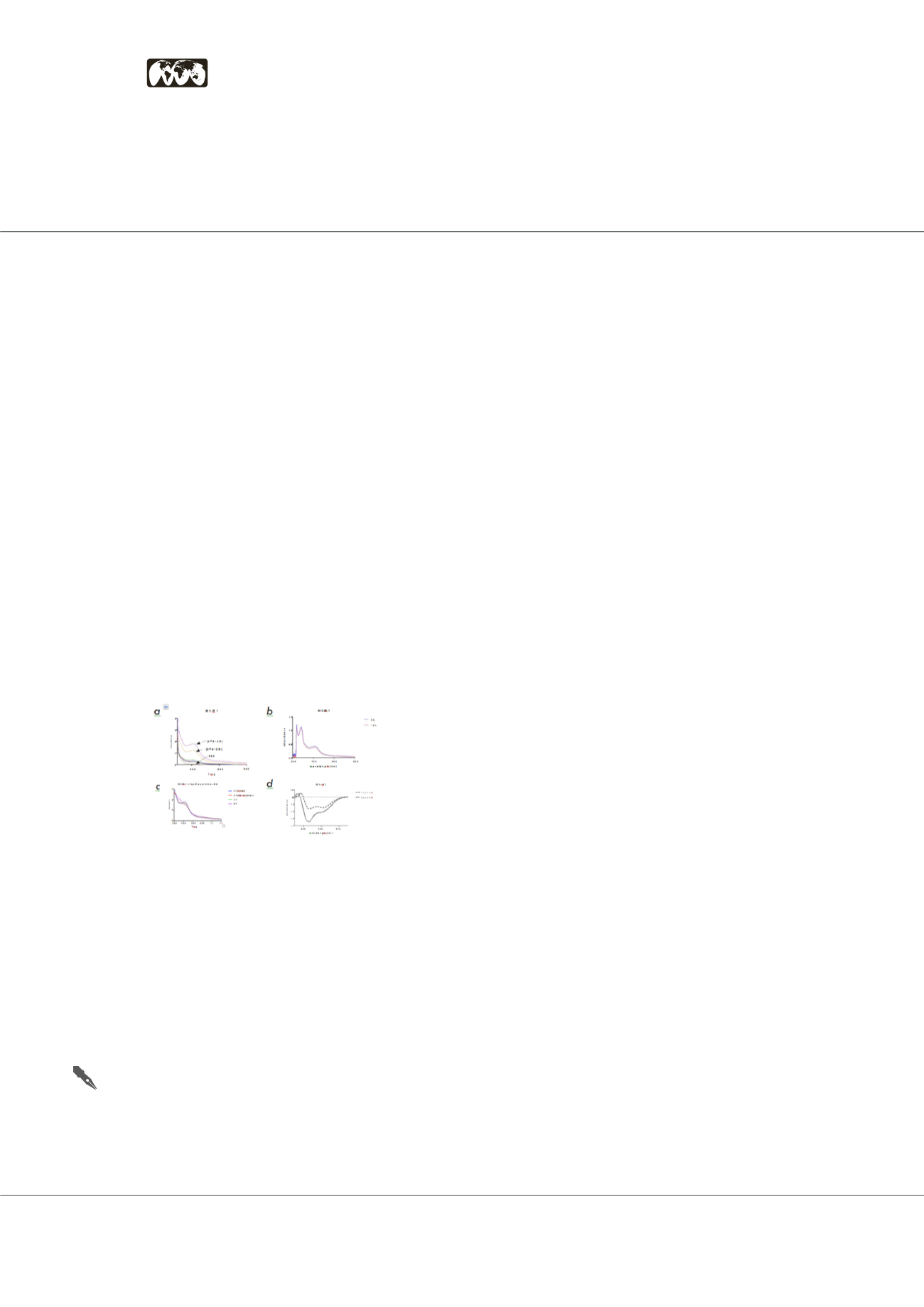
The study figure. Isolation and effect of [4Fe-4S] cluster
on the oligomeric state of K. sedentarius MBB WhiB1. (a)
Isolation of WhiB1 in three forms (indicated in black arrows).
(b) UV-visible spectra were obtained before and after
exposing the WhiB1 [4Fe-4S] cluster to air, the absorbance at
420nm indicates that the iron-sulfur cluster did not degrade
under aerobic conditions. (c) UV-visible spectral changes
upon reaction of holo-WhiB1 with NO. (d) Far-UV circular
dichroism (CD) spectroscopy analysis of apo- and holo-
WhiB1, indicating that apo forms a feature at 204nm, while
holo forms two features at 218-222nm.
Speaker Biography
Meshari A Alhadlaq has received his BSs and MSs in Molecular biology from Qassim and
Bangor universities in Saudi Arabia and United Kingdom in 2007 and 2013 respectively.
Then he joined the molecular biology and biotechnology department at the University of
Sheffield as a PhD candidate in 2015, to study the structure and biochemistry of protein.
Since then his studies focus on the characterisation and role ofWhiB proteins of Kytococcus
sedentarius.
e:
maalhadlaq1@sheffield.ac.ukMeshari A Alhadlaq
, Res Rep Gynaecol Obstet, Volume 3
DOI: 10.4066/2591-7366-C1-003
















