Research Article - Biomedical Research (2016) Volume 27, Issue 4
Zinc supplementation ameliorates ER stress and autophagy in liver in a rat model of type 2 diabetes mellitus
Meihua Piao1, Ya Liu2, Ting Yu3 and Ying Lu4*
1Department of Anesthesiology, First Hospital of Jilin University, Changchun, Jilin 130021, PR China
2School of Public Health, Jilin University, Changchun, Jilin 130021, PR China
3Department of Nutrition, Second Hospital of Jilin University, Changchun, Jilin 130041, PR China
4Intensive Care Unit, First Hospital of Jilin University, Changchun, Jilin130021, PR China
Accepted date: March 26, 2016
Abstract
This article explored the protective effects of zinc supplementation on diabetic liver injury and studied the underlying mechanisms that zinc supplementation reduced ER stress and autophagy in diabetic liver. Type 2 diabetes mellitus-like Wistar rat models were intragastrically administrated with ZnSO4 15 mg/kg daily for 53 days. Liver changes were examined by biochemical assay of serum, histopathological assay, immunohistochemical assay, radioimmunoassay and Western blotting. In results, compared with diabetes model control, zinc supplementation reduced the lipid peroxidation MDA levels, and enhanced the anti-oxidation T-AOC levels (P<0.05). H&E staining showed that pathological changes in diabetic liver were improved by zinc supplementation. Zinc supplementation enhanced MT protein expression in diabetic liver. In immunohistochemical assay, the amount of p-Akt positive cells was apparently increased, and the amount of LC3-II positive cells were lowered. Western blots of autophagy-associated LC3-II protein and endoplasmic reticulum(ER) stress-associated GRP78 proteins were lowered following a long-term zinc supplementation. In conclusion, zinc supplementation enhanced MT protein synthesis and Akt/PKB phosphorylation, which relieve GRP78-linked ER stress and LC3-II-linked autophagy. Zinc supplementation improved liver conditions in T2DM rat models through multiple pathways, in which GRP78-linked ER stress and LC3-II-linked autophagy are ameliorated to some degree. This finding is conducive to understanding the mechanisms that zinc supplementation prevent diabetic liver injury.
Keywords
Zinc; Diabetes mellitus, Metallothionien, Protein kinase B, ER stress, Autophagy.
Introduction
Type 2 diabetes mellitus (T2DM) is a common chronic metabolic disease worldwide [1-3], in which there are high blood glucose levels over a prolonged period, with multiple organ damage. Pathogenesis of T2DM is involved with multiple factors that are closely related to long-term high-fat or high-sugar diet, and genetic factors [4]. The pathological mechanism of T2DM involves β cell dysfunction and insulin resistance (IR), resulting in absolute or relative lack of insulin secretion. Deficiency of zinc over time may affect insulin activity leading to IR in T2DM [5]. Zinc supplementation in T2DM patients reduces oxidative stress by modulating the glutathione metabolism and metallothionein expression and promotes phosphorylation of insulin receptors by enhancing transport of glucose into cells [6-8]. Zinc-binding protein Metallothioneins (MTs) play an important role as potent antioxidants in the process that zinc prevents various oxidative damages in T2DM [9-12]. In addition, MTs prevent type 1 DM-induced oxidative damage, ER stress, cell death and trace element imbalances in liver following Zinc supplementation in OVE26 mouse model of type 1 DM [10].
Serine/threonine protein kinase B (PKB/Akt) mediates PI3Kcontrolled insulin signaling events that Akt upregulates quickly as the insulin signal pathway is stimulated by insulin [13-15]. ER stress plays an important role in the pathogenesis of diabetes to worsen islet β-cell loss and insulin resistance [16-19]. Elevated levels of molecular chaperone glucoseregulated protein78 (GRP78/Bip) in ER are positive responses to ER stress in cells, thus GRP78 can serve as an indicator for ER stress.
Autophagy is a featured phenomenon in eukaryotic cells, known as type II programmed cell death. Autophagic vacuoles and microtubule-associated protein light chain 3 (LC3-II) levels are proportional in mammalian cells, thus we can determine autophagy status by detecting changes in LC3-II content [20]. Liver damage often happens in patients with DM because of oxidative damage, endoplasmic reticulum(ER) stress and lipid peroxidation [21,22]. However, the relation between MT and Akt in liver in T2DM, or the impact of MT on ER stress and autophagy in liver in T2DM remains unclear.
This article explores MT and Akt expression in T2DM rat models, and how MT expression affects ER stress or autophagy by detecting GRP78 or LC3-II levels.
Materials and Methods
Animal modeling and treatment
Totally 39 male Wistar rats weighing 180-220 g were kept at a constant temperature 22 ± 2°C in The Laboratory Animal Center of Jilin University. The animal experiments were approved by The Jilin University Animal Ethics Committee. Type 2 diabetes mellitus(T2DM)-like rat models were prepared by a single intraperitoneal administration of streptozocin (STZ, 40 mg/kg weight) following 35-days high-fat dieting as referenced to the literature [23]. Three days following STZ injection, the fasting blood-glucose (FPG) was tested that FPG ≥ 16.7 mmol/l was diagnosed to be T2DM [24]. Subsequently, for the DM+Zn (diabetes with Zn supplementation) group, T2DM rats underwent intragastric (ig) administration of 15 mg ZnSO4 in 5 ml double-distilled water per kilogram bodyweight daily until sacrificed. Diabetes-free normal control (NC) and T2DM model control (MC) groups were treated with equivalent volumes of double-distilled water. Rats in the NC group always took normal diet, and rats in the DM+Zn group and the MC group always took high-fat diet.
The body weight was recorded every 7 days. At 53 days following Zn supplementation, rats were narcotized by intraperitoneal injection of 3% pentobarbital sodium at 30 mg/kg weight, and then fixed at the supine position. Blood was collected by abdominal vein. The whole blood was placed at 4°C for 1.5h, and then centrifuged at 3,000 ×g for 15 min at 4°C to obtain serum for inspection. Livers were removed and placed rapidly into precooled 0.9% NaCl solution at 4°C for rinsing off the blood. After dried by filter papers, livers were weighed and assayed.
Biochemical assay of serum
Automatic biochemical analyzer (7170A, Hitachi Inc., Tokyo, Japan) was used to test the FPG, alanine aminotransepherase (ALT) and aspartate aminotransepherase (AST). Serum insulin was assayed by a radioimmunoassay method using the Insulin Radioimmunoassay Kit (Northern Institute of Biotechnology, Beijing, China) according to the manufacture’s instruction. The radioactivity was detected by SN-695 gamma radioimmune counter (Hesuo Rihuan Photoelectric Instrument Co, LTD, Shanghai, China).
Histological staining
Livers were cut into pieces, and fixed in 10% neutral formaldehyde solution for histological and immunohistochemical examinations. Fixed liver pieces were embedded in paraffin, sectioned into slices of 4 μm thick, dewaxed by xylene, and dehydrated by a series of graded ethanol. A portion of dehydrated slices were conventionally stained with hematoxylin and eosin (H&E). For immunohistochemical assays on phosphorylated Akt/PKB (p- Akt) and LC3-II, dehydrated slices were placed in 3% H2O2 for 10 min to block endogenous peroxidase, and boiled 10 min in 0.01 mol/l sodium citrate buffer (pH=6.0). Then, slices on the slides were blocked 15 min with anti-species serum following washing by 1 mol/l PBS (pH=7.4). Goat anti-rat p- Akt and anti-rat LC3-II polyclonal antibodies (Beyotime Institute of Biotechnology, Najing, Jiangsu, China) were added at 4°C overnight. Biotinylated secondary antibody rabbit anti-goat IgGs were added for 20 min at 37°C. Streptavidin and biotinylated horseradish peroxidase were added for 20 min at 37°C, followed by color development using the DAB (3,3- diaminobenzidine) kit (Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China). Brown stains indicated positives. Microscopic examination (Olympus PM-10A0, Olympus, Beijing, China) was done by two separately blind pathologists. Each pathologist count the positive cells from randomly selected 5 microscopic fields (400X) of each slice and calculated their percentage: <5%, 525%, 2550% and 5075% graded ±, +, ++ and +++, respectively.
Detection of tissue homogenates
Livers were put in precooled 10 mmol/l Tris-HCl buffer (pH=7.4, at 4°C) with a mass/volume ratio of 1 g/4 ml, homogenized on ice bath, and centrifuged at 10,000 ×g for 10 min at 4°C. Lipid peroxidation product MDA in supernatants was assayed by the thiobarbituric acid (TBA) method [25] using the MDA detection kit (Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China) according to the manufacture’s instructions. Total anti-oxidation capacity (TAOC) was also detected using the T-AOC detection kit (Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China). MT protein content was determined by the 109Cd hemoglobin saturation method as referenced to the literature [26]. Briefly, supernatants were boiled for 2 min, and centrifuged at 10,000 ×g for 2 min to removed heat-precipitated proteins. An aliquot of 200 μl supernatants were transferred into an Eppendorf tube, and 200 μl of radioactive cadmium (2.0 mg 109CdCl2/ml with radioactivity of 1.0 μCi/ml from Sigma, USA) was added. The mixture was incubated for 10 min at room temperature. Free cadmium was thoroughly removed by a repeated procedure of 2% bovine hemoglobin binding. The gamma-ray radioactivity of cadmium-binding MT was assayed using Wizard 1470 Gamma Counter (Perkin-Elmer Corporation, Shanghai, China). MT protein concentration was calculated by a ratio of 1 μmol MT to 6 μmol cadmium.
LC3-II and GRP78 levels were detected by Western blotting assay. Briefly, total protein was extracted using RIPA lysis buffer. Protein concentrations were determined using the BCA Protein Assay Kit (Pierce Chemical Company, Rockford, IL) according to the manufacturer’s instructions. Proteins were separated by 12% (w/v) SDS-PAGE and electroblot to PVDF membranes. Membranes were blocked for 2 h in TBS buffer (10 mM Tris-HCl, 150 mM NaCl, 1 vol. % Tween 20) containing 5% skimmed milk powder and 0.5% BSA at room temperature. Anti-GRP78 and anti-LC3-II polyclonal antibodies (Beyotime Institute of Biotechnology, Nanjing, Jiangsu, China) were used with a dilution 1:1000 at 4°C overnight, respectively. After washed by TBS buffer, membranes were incubated with horseradish peroxidase(HRP)- conjugated secondary antibody (diluted by 1:1000. Zhongshan GoldenBridge Biotechnology Co., Ltd., Beijing, China) for 1h at room temperature. Color was developed using an enhanced chemiluminescence(ECL) detection kit (Pierce Chemical Company, Rockford, IL). β-actin was inner reference. Relative blot grayscales were determined by a ratio of test protein to β- actin grayscales using UVP BioImaging and Analysis Systems (UVP, LLC, Upland, CA).
Statistical analyses
SPSS12.0 software was used for statistical analyses. Data were expressed as mean ± SEM. One-way ANOVA with Dunn’ ad hoc test and Wilcoxon rank sum test (Mann–Whitney U test) were used to compare between multiple groups. P<0.05 was statistical significance.
Results
General data
High-fat diet feeding for 35 days prepared hyperlipemia successfully in rats. Then, three days following STZ injection, FPG was detected. The rats with FPG ≥ 16.7 mmol/l were used in the subsequent experiments.
Finally, 15 T2DM-like rats were qualified, 7 and 8 rats for the MC (diabetes model control) and DM+Zn (diabetes with Zn supplementation) groups, respectively. Nine rats served as the diabetes-free normal control (NC). General data of rats including body weight, blood glucose and insulin were shown in table 1.
| n | Body weight (g) | FPG (mmol/l) | Insulin (μIU/ml) | |||
| Day 4 | Day 53 | Day 0 | Day 53 | Day 53 | ||
| NC | 9 | 320 ± 6 | 401 ± 8 | 3.6 ± 0.7 | 3.9 ± 0.6 | 30.4 ± 1.7 |
| MC | 7 | 278 ± 8 a | 320 ± 7 a | 25.2 ± 1.6 a | 26.6 ± 1.9 a | 20.8 ± 1.8 a |
| DM+Zn | 8 | 300 ± 9 a,b | 378 ± 6 a,b | 23.8 ± 1.9 a | 11.9 ± 1.4 a,b | 26.1 ± 1.3 a,b |
| a P<0.05 vs. the NC group, b P<0.05 vs. the MC group. NC, normal control (n=9); MC, diabetes model control (n=7); DM+Zn, diabetes with Zn supplementation (n=8). | ||||||
Table 1. Body weight, FPG and insulin at different time points after Zn supplement started.
Liver mass, function and redox indices
Liver mass (liver/body weight), liver function (AST/ALT), lipid peroxidation (MDA) and anti-oxidation (T-AOC) indices at 53 days following Zn supplementation were shown in table 2. Upon liver mass, AST/ALT levels and MDA levels, the MC and DM+Zn groups were significantly higher than the NC group (P<0.05). These indices in the DM+Zn group were reduced a bit compared with the MC group (P<0.05 or P>0.05). Anti-oxidation T-AOC levels in the MC and DM+Zn groups were significantly lower than the NC group (P<0.05). And T-AOC levels in the DM+Zn group were enhanced significantly compared with the MC group (P<0.05).
| n | Liver/body weight (mg/g) | AST (U/l) | ALT (U/l) | MDA (nmol/ml) | T-AOC (U/ml) | |
| NC | 9 | 27.8 ± 2.7 | 113 ± 17 | 45 ± 12 | 4.1 ± 0.6 | 10.7 ± 0.9 |
| MC | 7 | 58.2 ± 3.9 a | 186 ± 22 a | 88 ± 14 a | 5.9 ± 0.8 a | 5.9 ± 0.9 a |
| DM+Zn | 8 | 42.9 ± 3.6a | 141 ± 19 a | 67 ± 16 a | 4.8 ± 0.7 a,b | 9.4 ± 1.1 a,b |
| a P<0.05 vs. the NC group, b P<0.05 vs. the MC group. NC, normal control (n=9); MC, diabetes model control (n=7); DM+Zn, diabetes with Zn supplementation (n=8). | ||||||
Table 2. Liver mass, function and redox indices 53 days after Zinc supplementation.
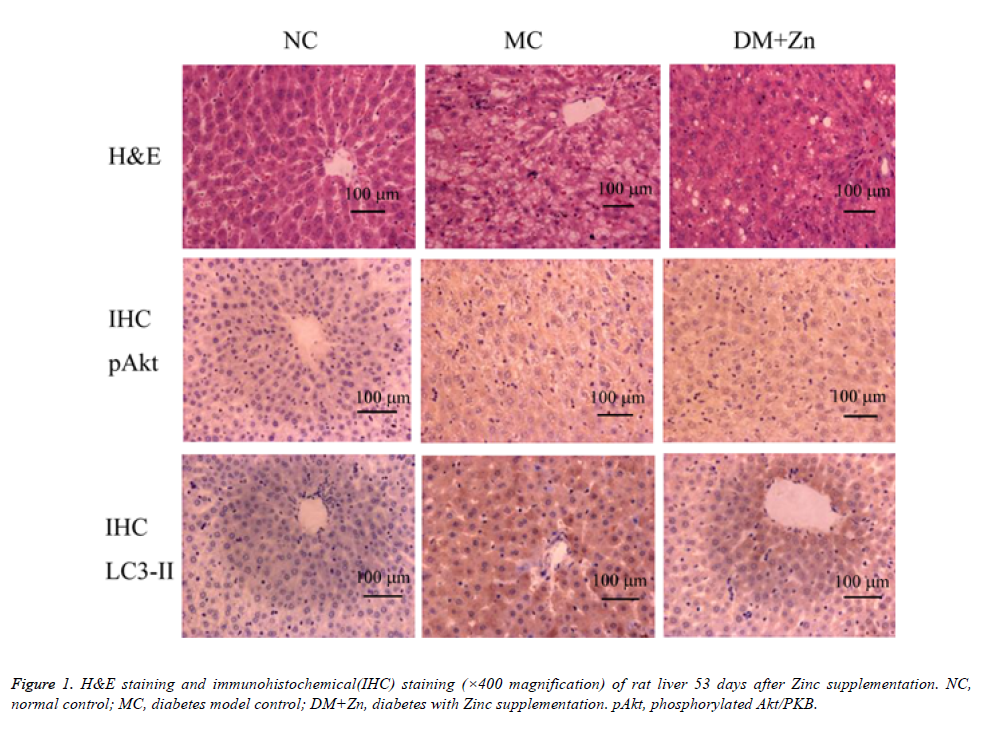
Liver H&E staining
Pathological changes in liver tissues were observed under the microscopic examination (see Figure 1). Upon the NC group, lobules were integral in structure, liver cells were arranged in order, nuclei were located in the center of cells, and cytoplasmic staining was evenly. Small vacuolar fat droplets were occasionally seen, and lymphocyte infiltration was not seen. Upon the MC group, lobules were irregular, and liver cells were disordered with severe damage. Severe fatty degeneration and dot-like or focal necrosis were seen. Parts of nuclei were extruded from the central position. Lobules were atropic or disappeared. Lymphocyte infiltration was seen in parts of liver tissues. Upon the DM+Zn group, liver cells were arranged irregularly, and parts of them were less structured with vacuolar fat droplets. Severe fatty degeneration was not seen but a bit dot-like necrosis.
Liver MT content detected by 109Cd hemoglobin saturation method
MT content was detected by the 109Cd hemoglobin saturation method. MT content of the MC group was 12.2 ± 1.5 μg/g liver, significantly higher than 8.6 ± 0.6 μg/g of the NC group (n=5, P<0.05). The DM+Zn group 38.1 ± 4.7 μg/g was significantly higher than the MC group (n=5, P<0.05).
Immunohistochemical staining of liver p-Akt and LC3-II
Table 3 showed the immunohistochemical grade of positive liver cell percentages 53 days after Zn supplementation. The staining images were shown in figure 1. In results, the amount of p-Akt positive cells in the MC and DM+Zn groups was significantly more than the NC group (P<0.05), and that in the DM+Zn group was significantly more than the MC group (P<0.05). The amount of LC3-II positive cells in the MC and DM+Zn groups was significantly more than the NC group (P<0.05), and that in the DM+Zn group was significantly lower than the MC group (P<0.05).
| Grade | ± | + | ++ | +++ | |
| p-Akt | NC | 28 | 2 | 0 | 0 |
| MC a | 9 | 14 | 7 | 0 | |
| DM+Zn a,b | 0 | 5 | 14 | 11 | |
| χ2=144.8, p=0.001 | |||||
| LC3-II | NC | 30 | 0 | 0 | 0 |
| MC a | 0 | 11 | 18 | 1 | |
| DM+Zn a,b | 8 | 18 | 4 | 0 | |
| χ2=107.1, p=0.001 | |||||
| Randomly 5 microscopic viewfields of each slice, 6 slices for each group. aP<0.05 vs. the NC group, bP<0.05 vs. the MC group. NC, normal control (n=9); MC, diabetes model control (n=7); DM+Zn, diabetes with Zn supplementation (n=8). | |||||
Table 3. Immunohistochemical grade of positive liver cell percentages of p-Akt and LC3-II 53 days after Zinc supplementation.
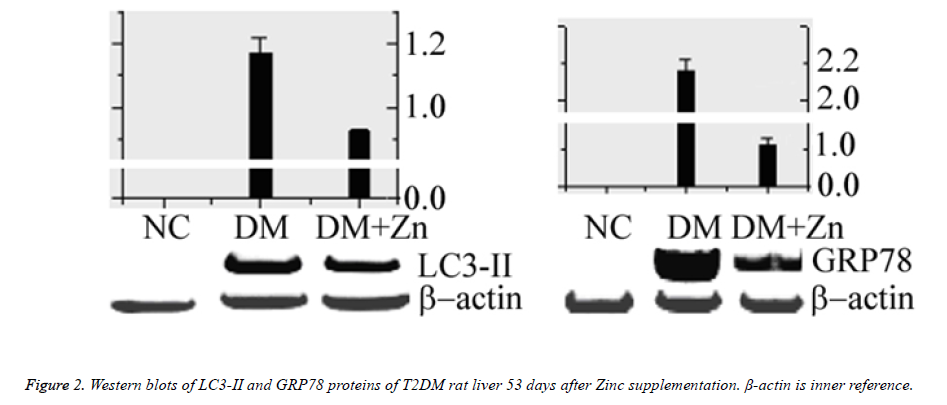
Western blots of GRP78 and LC3-II
GRP78 and LC3-II protein blots were shown in figure 2. The NC group had no blot signal. Both relative blot grayscales of GRP78 and LC3-II proteins in either the MC group or the DM +Zn group were significantly higher than the NC group (n=3, P<0.05), and the DM+Zn group significantly lower than the MC group (n=3, P<0.05).
Discussion
Oxidative stress exists widely in the development of diabetes and its complications, with sustained high blood glucose and increased free radicals, causing oxidative damage to tissues. Hazardous substances attack liver cell membranes, resulting in lipid peroxidation, forming lipid peroxidation product MDA. Then, lipid peroxidation elevates reactive oxygen species (ROS) that lead to metabolic disorders in cells, even death. In the current study, blood glucose levels were increased and insulin levels were lowered in T2DM-like rats, indicating that the high-fat diet feeding and injection of STZ induced T2DMlike rat models. Compared with the normal control, T2DM-like rats had an increase in liver mass index, liver function (AST/ ALT) levels and lipid peroxidation (MDA) levels, or a decrease in anti-oxidation T-AOC levels, or presented a worsened histological necrosis. Zinc supplementation improved these conditions. Compared with the MC control, zinc supplementation improved insulin levels and reduced blood glucose levels; reduced liver mass index and liver function, reduced lipid peroxidation MDA, enhanced anti-oxidation TAOC levels, and improved the histological necrosis.
MT has a protective effect on DM because of its anti-oxidation effect, and MT molecules are the core nodes that link intracellular zinc to redox-mediated signal transduction [10,22]. These features are important for prevention and mitigation of diabetes. In the current study, zinc supplementation in the DM+Zn group promoted MT synthesis, reduced oxidative stress, and improved weight loss in T2DM rats. Zinc supplementation upregulated MT expression that scavenge oxygen free radicals, subsequently enhanced the antioxidant effect and reduced lipid peroxidation, and finally prevented oxidative damage in diabetic liver.
In the current study, Akt phosphorylation was kept at a low level in normal liver cells (the NC group). Akt phosphorylation was upregulated a little in the STZ-treated MC group because of diabetic oxidative stress. Because zinc is insulinomimetic [27], zinc supplementation upregulated markedly pAkt levels in diabetic liver in the DM+Zn group compared with the MC group (P<0.05). At the same time, zinc-induced MT upregulation was also potent to activate Akt in diabetic liver cells [28-30].
ER stress is related to the pathogenesis of diabetes and GRP78 can serve as indicator for ER stress [11,12]. In the current study, rats in the MC group showed high GRP78 blots, demonstrating that severe ER stress happened in liver cells in T2DM rats. Levels of GRP78 blots were reduced in liver in the Zinc-supplemented DM+Zn group compared with the MC group, but still higher than the normal control, demonstrating that zinc supplementation is conducive to ameliorate ER stress in diabetic liver partially. Studies have shown that Akt are involved in the T2DM-associated ER stress through three signaling pathways IRE-1α-JNK [31,32], CHOP [33] and GSK3β [34]. In the current study, zinc supplementation upregulated MT expression in liver, subsequently and/or simultaneously activated Akt, thus reduced ER stress in diabetic liver.
LC3-II content indicates autophagy status in cells. In the current study, the immunohistochemical results showed that autophagy happened severely in liver of T2DM rats in the MC group, zinc supplementation (DM+Zn group) downregulated LC3-II levels significantly than the MC group, but still higher than the normal control, demonstrating that zinc supplementation reduced autophagy in diabetic liver partially. The results of Western blotting obtained the consistent outcomes. Exactly, p-Akt is reported to involve also in the autophagy pathways [35,36]. Thus in the present study, zinc supplementation upregulated MT and/or p-Akt levels, and downregulated LC3-II levels, demonstrating that MT and/or p- Akt may ameliorate autophagy in diabetic liver.
Hereby, two molecular mechanisms that improve liver conditions by zinc supplementation in T2DM rat models were as follows. (1) Zinc supplementation promoted MT synthesis to reduce oxidative stress in diabetic liver, and subsequently ameliorated GRP78-linked ER stress and LC3-II-linked autophagy. (2) Zinc supplementation also promoted Akt phosphorylation to suppress GRP78-linked and/or LC3-IIlinked autophagy. Therefore, improved liver conditions in T2DM rat models in the current study were the results from regulation of multiple pathways by zinc supplementation, including MT and p-Akt upregulation and/or GRP78 and LC3- II downregulation. Zinc supplementation improved liver conditions in T2DM rat models through multiple pathways, in which GRP78-linked ER stress and LC3-II-linked autophagy are ameliorated to some degree.
Conflict of Interest:
The authors declare that they have no conflict of interest.
References
- Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, Shan Z, Liu J, Tian H, Ji Q, Zhu D, Ge J, Lin L, Chen L, Guo X, Zhao Z, Li Q, Zhou Z, Shan G, He J. New England J Med 2010; 362: 1090-1101.
- Pandya N, Wei W, Meyers JL, Kilpatrick BS, Davis KL. Burden of sliding scale insulin use in elderly long term care residents with type 2 diabetes mellitus. J Am Geriatr Soc. 2013; 61: 2103-2110.
- Li ZP, Zhang M, Gao J, Zhou GY, Li SQ, An ZM. Relation between ADIPOQ Gene Polymorphisms and Type 2 Diabetes. Genes (Basel) 2015; 6: 512-519.
- Wang WL, Zhu H, Xie Y, Li J. Relation between ADIPOQ gene polymorphisms and type 2 diabetes in a Chinese population. Int J Clin Exp Med. 2015; 8: 6124-6128.
- Myers SA. Zinc transporters and zinc signaling: new insights into their role in type 2 diabetes. Int J Endocrinol. 2015; 2015: 167503.
- Cruz KJ, de Oliveira AR, Marreiro Ddo N. Antioxidant role of zinc in diabetes mellitus. World J Diabetes 2015 Mar 15; 6: 333-337.
- Kloubert V, Rink L. Zinc as a micronutrient and its preventive role of oxidative damage in cells. Food Funct 2015; 6: 3195-3204.
- Sacan O, Turkyilmaz IB, Bayrak BB, Mutlu O, Akev N, Yanardag R. Zinc supplementation ameliorates glycoprotein components and oxidative stress changes in the lung of streptozotocin diabetic rats. Biometals 2016.
- Ganasyam SR, Rao TB, Murthy YS, Jyothy A, Sujatha M. Association of Estrogen Receptor-α Gene & Metallothionein-1 Gene Polymorphisms in Type 2 Diabetic Women of Andhra Pradesh. Indian J Clin Biochem 2012; 27: 69-73.
- Liang T, Zhang Q, Sun W, Xin Y, Zhang Z, Tan Y, Zhou S, Zhang C, Cai L, Lu X, Cheng M. Zinc treatment prevents type 1 diabetes-induced hepatic oxidative damage, endoplasmic reticulum stress, and cell death, and even prevents possible steatohepatitis in the OVE26 mouse model: Important role of metallothionein. Toxicol Lett 2015; 233: 114-124.
- Xu J, Wang G, Wang Y, Liu Q, Xu W, Tan Y, Cai L. Diabetes- and angiotensin II-induced cardiac endoplasmic reticulum stress and cell death: metallothionein protection. J Cell Mol Med. 2009; 13: 1499-1512.
- Sato M, Suzuki S. Endoplasmic reticulum stress and metallothionein. Yakugaku Zasshi. 2007; 127: 703-708.
- Yang M, Ren Y, Lin Z, Tang C, Jia Y, Lai Y, Zhou T, Wu S, Liu H, Yang G, Li L. Krüppel-like factor 14 increases insulin sensitivity through activation of PI3K/Akt signal pathway. Cell Signal. 2015; 27: 2201-2208.
- Schultze SM, Hemmings BA, Niessen M, Tschopp O. PI3K/AKT, MAPK and AMPK signalling: protein kinases in glucose homeostasis. Expert Rev Mol Med 2013; 14: e1.
- Hu X, Wang S, Xu J, Wang D-B, Chen Y, Yang G-Z. Triterpenoid saponins from stauntonia chinensis ameliorate insulin resistance via the AMP-activated protein kinase and IR/IRS-1/PI3K/Akt pathways in insulin-resistant HepG2 cells. Int J Mol Sci 2014; 15: 10446-10458.
- Cunard R. Endoplasmic reticulum stress in the diabetic kidney, the good, the bad and the ugly. J Clin Med 2015; 4: 715-740.
- Wali JA, Masters SL, Thomas HE. Linking metabolic abnormalities to apoptotic pathways in beta cells in type 2 diabetes. Cells 2013; 2: 266-283.
- Liu S, Li K, Li T, Xiong XD, Yao S, Chen Z, Wang C, Zhao B. Association between promoter polymorphisms of the GRP78 gene and risk of type 2 diabetes in a Chinese han population. DNA Cell Biol 2013; 32: 119-124.
- Zhang Q, Li Y, Liang T, Lu X, Zhang C, Liu X, Jiang X, Martin RC, Cheng M, Cai L. ER stress and autophagy dysfunction contribute to fatty liver in diabetic mice. Int J Biol Sci. 2015; 11: 559-568.
- Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol 2004; 36: 2503-2518.
- Asakawa M, Mitsui H, Akihisa M, Sekine T, Niitsu Y, Kobayashi A, Miyake A, Hashimoto N, Kawamura M, Ogawa Y. Efficacy and safety of sitagliptin for the treatment of diabetes mellitus complicated by chronic liver injury. Springerplus 2015; 4: 346.
- Ortiz-Avila O, Gallegos-Corona MA, Sánchez-Briones LA, Calderón-Cortés E, Montoya-Pérez R, Rodriguez-Orozco AR, Campos-García J, Saavedra-Molina A,>Mejía-Zepeda R, Cortés-Rojo C. Protective effects of dietary avocado oil on impaired electron transport chain function and exacerbated oxidative stress in liver mitochondria from diabetic rats. J Bioenerg Biomembr 2015; 47: 337-353.
- Lu Y, Liu Y, Li H, Wang X, Wu W, Gao L. Effect and mechanisms of zinc supplementation in protecting against diabetic cardiomyopathy in a rat model of type 2 diabetes. Bosn J Basic Med Sci 2015; 15: 14-20.
- Li Y, Gu Y, Song Y, Zhang L, Kang YJ, Prabhu SD. Cardiac functional analysis by electrocardiography, echocardiography and in situ hemodynamics in streptozotocin-induced diabetic mice. J Health Sci 2004; 50: 356-365.
- Esterbauer H, Zollner H. Methods for determination of aldehydic lipid peroxidation products. Free Radic Biol Med 1989; 7: 197-203.
- Hidalgo J, Penkowa M, Giralt M, Carrasco J, Molinero A. Metallothionein expression and oxidative stress in the brain. Methods Enzymol 2002; 348: 238-249.
- Roussel AM, Kerkeni A, Zouari N, Mahjoub S, Matheau JM, Anderson RA. Antioxidant effects of zinc supplementation in Tunisians with type 2 diabetes mellitus. J Am Coll Nutr 2003; 22: 316-321.
- Asmussen JW, Ambjorn M, Bock E, Berezin V. Peptides modeled after the alpha-domain of metallothionein induce neurite outgrowth and promote survival of cerebellar granule neurons. Eur J Cell Biol 2009; 88: 433-443.
- Dong F, Li Q, Sreejayan N, Nunn JM, Ren J. Metallothionein prevents high-fat diet induced cardiac contractile dysfunction:role of peroxisome proliferator activated receptor gamma coactivator 1alpha and mitochondrial biogenesis. Diabetes 2007; 56: 2201-2212.
- Urso ML, Scrimgeour AG, Chen YW, Thompson PD, Clarkson PM. Analysis of human skeletal muscle after 48 h immobilization reveals alterations in mRNA and protein for extracellular matrix components. J Appl Physiol 2006; 101: 1136-1148.
- Muoio DM, Newgard CB. Biomedicine. Insulin resistance takes a trip through the ER. Science 2004; 306: 425-426.
- Hartman MG, Lu D, Kim ML, Kociba GJ, Shukri T, Buteau J, Wang X, Frankel WL, Guttridge D, Prentki M, Grey ST, Ron D, Hai T. Role for activating transcription factor 3 in stress-induced beta-cell apoptosis. Mol Cell Biol 2004; 24: 5721-5732.
- Ishihara H, Takeda S, Tamura A, Takahashi R, Yamaguchi S,Takei D, Yamada T, Inoue H, Soga H, Katagiri H, Tanizawa Y, Oka Y. Disruption of the WFS1 gene in mice causes progressive β-cell loss and impaired stimulus-secretion coupling in insulin secretion. Hum Mol Genet 2004; 13: 1159-1170.
- Srinivasan S, Ohsugi M, Liu Z, Fatrai S, Bernal-Mizrachi E, Permutt MA. Endoplasmic reticulum stress-induced apoptosis is partly mediated by reduced insulin signaling through phosphatidylinositol 3-kinase/Akt and increased glycogen synthase kinase-3beta in mouse insulinoma cells. Diabetes 2005; 54: 968-975.
- Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ 2015; 12: 1509-1518.
- Scarlatti F, Bauvy C, Ventruti A, Sala G, Cluzeaud F, Vandewalle A, Ghidoni R, Codogno P. Ceramide-mediated macroautophagy involves inhibition of protein kinase B and up-regulation of beclin 1. J Biol Chem 2004; 279: 18384-18391.