Research Article - Biomedical Research (2017) Volume 28, Issue 9
Xuefu Zhuyu decoction induces the proliferation, migration, adhesion of human umbilical vein endothelial cells via VEGF-VEGFR2 pathway
Jiawei Yang#, Wenjun Guan# and Pei Hu*Jingzhou Central Hospital of Cardiology, the Second Clinical Medical College, Yangtze University, JinZhou, JingZhou, PR China
#These authors contributed equally to this work
- *Corresponding Author:
- Pei Hu
Jingzhou Central Hospital of Cardiology
The Second Clinical Medical College
Yangtze University, PR China
Accepted on January 27, 2017
Abstract
Aims: The present study aims to assess the effect of Xuefu Zhuyu Decoction (XFZYD) in promoting the proliferation, migration and adhesion of endothelial cells, and to investigate the preliminary mechanisms.
Methods: Human Umbilical Vein Endothelial Cells (HUVECs) were incubated with Blank Serum (B-S) and XFZYD-Containing Serum (XFZYD-CS) at final concentrations of 1.25%, 2.5% and 5% for 48 h. The proliferation, migration and adhesion of HUVECs were assessed by Methyl Thiazolyl Tetrazolium (MTT) assay, Boyden chamber assay and gelatin adhesion assay, respectively. Secretion of Vascular Endothelial Growth Factor (VEGF) was evaluated by Enzyme-Linked Immunosorbent Assay (ELISA). Synthesis of intracellular VEGF and Vascular Endothelial Growth Factor Receptor-2 (VEGFR2) was studied by immunohistochemistry. VEGF and VEGFR2 mRNA expression was evaluated by quantitative real-time polymerase chain reaction (qRT-PCR).
Results: The data showed that 1.25% XFZYD-CS had no effect on HUVECs activity; 2.5% XFZYD-CS promoted the proliferation, migration and adhesion of HUVECs; 5% XFZYD-CS enhanced the migration, adhesion and intracellular VEGF expression in HUVECs.
Conclusions: The present study demonstrates that XFZYD induces the proliferation, migration and adhesion of HUVECs through VEGF-VEGFR2 pathway, suggesting that the mechanism by which XFZYD stimulates angiogenesis in vivo may be these actions.
Keywords
Xuefu Zhuyu decoction, Human umbilical vein endothelial cells, VEGF-VEGFR2 pathway.
Introduction
Angiogenesis, the formation of new blood vessels from a preexisting vascular network, occurs in either physiological or pathological conditions [1]. This process is closely regulated by a series of pro- and anti-angiogenic molecules in normal physiology. However, serious consequences may occur when this equilibrium is broken [2], and abnormal angiogenesis participates in tumor development [3]. Stimulation of angiogenesis can be used to accelerate wound healing and to promote the growth of collateral blood vessels in ischemic tissues [4]. Angiogenesis is stimulated by a large number of pro-angiogenic molecules, among which Vascular Endothelial Growth Factor (VEGF) is one of the most important factors in angiogenesis [5]. VEGF exerts its activity in angiogenesis through binding to transmembrane endothelial cells VEGFR2 [6-8]. Binding of VEGF to VEGFR2 leads to intracellular receptor phosphorylation that initiates various intracellular downstream pathways, causing endothelial cell proliferation, migration and adhesion [8-11].
Xuefu Zhuyu Decoction (XFZYD) mentioned in Yilin Gaicuo (Correction on Errors in Medical Classics) is a popular traditional Chinese medicine that is frequently used for the treatment of ischemic diseases. It has been known that XFZYD possesses biological activities that prevent platelet aggregation and arteriosclerosis. It has been reported that XFZYD promotes angiogenesis in Chorioallantoic Membrane (CAM) model [12], facilitates endothelial progenitor cell tube formation and mobilizes bone marrow-derived endothelial progenitor cells for angiogenesis in rats [13]. However, its cellular and molecular mechanisms are even unknown. It is already known that VEGF-VEGFR2 pathway induces the proliferation, migration and adhesion of endothelial cells. Here, we explore cellular and molecular mechanisms on the basis of previous reports [14,15]. In this study, we investigate the effects of XFZYD on endothelial cell proliferation, migration, adhesion, as well as the underlying mechanisms.
Materials and Methods
Preparation of XFZYD
XFZYD was composed of Aaugellica sinensis (9 g), Rehmannia dride rhizome (9 g), semen persicae (12 g), flos carthami (9 g), fructus aurantii (6 g), radix paeoniae rubra (6 g), bupleurum root (3 g), radix glycytthizae (6 g), radix platycodi (4.5 g), rhizoma chuanxiong (4.5 g), and radix acanthopanacis bidentatae (9 g) (provided by Department of Pharmacy, General Hospital of Ningxia Medical University). Each drug was divided evenly into two portions and decocted separately. Then, the two portions of decoction were mixed and boiled to reach a concentration of 1.3 g crude drug/ml as described previously [13].
Animals
The XFZYD-containing serum was prepared as described previously [16]. Twelve 6-week old purebred Sprague-Dawley (SD) rats (Slac Laboratory Animal Co., Shanghai, China) were used in the present study. Half were males and half were females. All rats were in good health and each weighed 150 ± 20 g. The rats were maintained in certificated specific pathogen-free housing facility of the Experimental Animal Center of Ningxia Medical University. The temperature in the chamber was 22-24°C. All animals had 12 h light/dark cycle. All procedures were performed in accordance with institutional guidelines and conformed to the Guide for the Care and Use of Laboratory Animals as published by the National Institutes of Health (USA) and approved by the local Ethics Committee. Rats were randomly distributed into two groups. In the first group, each animal was orally administrated XFZYD at 13 g/kg twice a day for a total of seven doses. Blood was obtained from abdominal aorta 2 h after the last administration, and centrifuged at 4,000 rpm for 30 min. Serum was collected and termed as XFZYD-Containing Serum (XFZYD-CS). In second group, rats were orally administrated normal saline according to the same protocol, and the serum was used as Blank Serum (B-S). Both XFZYD-CS and B-S were inactivated by heating at 56°C for 30 min, filtered through 0.22 μm filter, and stored at -20°C until use.
Cells
Human umbilical vein endothelial cells (HUVECs) were obtained from China Center of Type Culture Collections, College of Life Sciences, Wuhan University, Wuhan, China, and grown in M199 medium (Thermo Fisher Scientific, Waltham, MA, USA) containing 5% fetal bovine serum at 37°C in an atmosphere with 5% CO2 as described previously [17]. When reaching confluence, HUVECs were detached using trypsin-EDTA solution. HUVECs were rendered synchronized by incubation for 24 h in serum-free M199 medium. Synchronized HUVECs were harvested and plated in 96-well plates for proliferation assay or 25 cm2 flasks for further assays at a density of 2.5 × 103 cells/well or 2.5 × 105 cells/flask in M199 medium supplemented with 5% fetal bovine serum. After 4 h, the medium was discarded. The cells were exposed to XFZYD-CS or B-S at final concentrations of 1.25%, 2.5% and 5% (w/w) for 48 h.
Methyl thiazolyl tetrazolium (MTT) assay
The effect of XFZYD-CS on HUVECs proliferation was estimated by MTT assay as described previously [17]. MTT (5 mg/ml; Amresco Co., Solon, OH, USA) was added to each well for incubation for 4 h. After MTT solution was replaced by 200 μl DMSO, the plates were shaken for 10 min. The optical density (OD) was assessed at 570 nm (reference wave, 630 nm) using a 96-well microplate reader (SynergyH1, BioTek, Winooski, VT, USA).
Boyden chamber assay
The migration of HUVECs was evaluated using Boyden chamber assay as described previously [18]. Boyden chamber (Haimen City Qilin Medical Instrument Factory, Jiangsu, China) was inserted with polycarbonate membrane. HUVECs treated with XFZYD-CS or B-S (2 × 104 cells) were suspended in 100 μl of corresponding culture medium and added to the upper chamber. The lower chamber was loaded with 100 μl corresponding cell culture supernatant. After incubation at 37°C for 1 h, residual cells in the upper side of the membrane were screwed with cotton swabs. Then, the membrane was fixed with 4% neutral formalin for 10 min. Cells that migrated to the lower side of the membrane were stained with haematoxylin. The stained cells were counted in 6 High Power (X400) Fields (HPF). Photographs were taken by an inverted phase contrast microscope (IX70, Olympus Co., Tokyo, Japan).
Gelatin adhesion assay
HUVECs adhesion assay was performed with gelatin adhesion assay described elsewhere [19]. Ninety-six-well plates were pre-coated with 1% gelatin. HUVECs (1 × 104 cells) treated with XFZYD-CS or B-S were incubated with 5% fetal bovine serum for 30 min. Then, culture medium was removed. Adherent cells from 6 fields (X100) were counted.
Enzyme-linked immunosorbent assay (ELISA)
VEGF concentration in cell culture supernatant was measured using human VEGF ELISA Kit according to the manufacturer’s protocols (Jingmei Biotech Co., Shenzhen, China).
Immunocytochemistry
Immunocytochemistry was performed as previously described [17]. Briefly, HUVECs treated with XFZYD-CS or B-S were washed with phosphate-buffered saline and fixed using 4% neutral formalin for 10 min. The cells were then permeabilized with 0.1% Triton X-100 in phosphate-buffered saline for 20 min at room temperature and washed with phosphate-buffered saline. Solution A and solution B were added according to the protocol of UltraSensitive S-P Kit (Maixin Biotech Co., Fuzhou, China). HUVECs were incubated at room temperature for 1 h in primary mouse anti-human VEGF instant monoclonal antibody or primary rabbit anti-human VEGFR2 instant polyclonal antibody (Maixin Biotech Co., Fuzhou, China). Then, solution C and solution D were added according to the protocols. The cells were then stained with 3-Amino-9- Eethyl-Carbazole (AEC) for 8 min at room temperature and examined under a microscope. Images were captured by Motic Images 2000 1.3 systems (Motic China Group Co., Ltd., Xiamen, China), and gray values were analyzed by Motic Med 6.0 systems (Motic China Group Co., Ltd., Xiamen, China) according to the manufacturer’s manual.
Quantitative real-time polymerase chain reaction (qRT-PCR)
qRT-PCR was performed as previously described [20]. Total RNA was obtained from HUVECs treated with XFZYD-CS or B-S using Trizol reagent, and the amount and purity of RNA were determined by spectrophotometry. Total RNA (1 μm) was reverse transcribed using Revertaid First Strand cDNA Synthesis Kit (Fermentas, Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocols and the resultant cDNA was amplified by PCR. Primers for VEGF were: 5’-TGG ATC CAT GAA CTT TCT GCT GTC-3’ (sense) and 5’-TCA CCG CCT TGG CTT GTC ACA T-3’ (antisense) (product size 452 bp); primers for VEGFR2 were 5’- GCACGATTCCGTCAAGGG-3’ (sense) and 5’- TTCAAAGGGAGGCGAGCA-3’ (antisense) (product size 383 bp); primers for β-actin were 5’-ATC ATG TTT GGG ACC TTC AAC A-3’ (sense) and 5’-CAT CTC TTG CTC GAA GTC CA-3’ (antisense) (product size 318 bp). PCR was performed in a thermal cycler (Perkin-Elmer, USA) under the following conditions: an initial denaturation at 94°C for 3 min, 35 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 45 s, followed by a final extension for 10 min. PCR products were analyzed with electrophoresis in TBE buffer stained with DNA green (Bioteke Biotech Co., Beijing, China) at 5V/cm for 40 min in 2.0% agarose gel. The level of gene expression was assessed by densitometric measurement of the amount of PCR products on scanned agarose gels (Genetech Co., Shanghai, China). The units of expression were calculated as the ratio of the amount of PCR product of studied mRNA to the amount of PCR product of the housekeeping gene β-actin, the amount of which was assumed to be expressed constantly in cells.
Statistical analysis
All results were analysed using SPSS 14.0 software (IBM, Armonk, NY, USA). All data were expressed as means ± SD. Differences between groups were analysed by Analysis of Variance (ANOVA) followed by Duncan’s multiple range test. P<0.05 was considered statistically significant.
Results
XFZYD promotes HUVECs proliferation
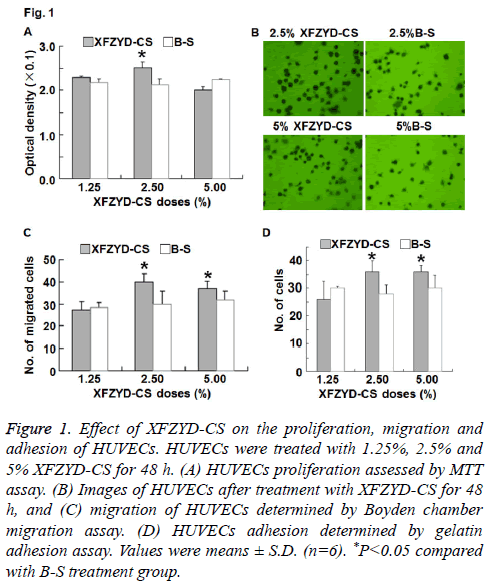
To detect the proliferation of HUVECs, MTT assay was used. The data showed that 2.5% XFZYD-CS increased HUVECs proliferation, whereas 1.25% and 5% XFZYD-CS failed to significantly alter HUVECs proliferation (Figure 1A). The result suggests that XFZYD promotes HUVECs proliferation.
Figure 1: Effect of XFZYD-CS on the proliferation, migration and adhesion of HUVECs. HUVECs were treated with 1.25%, 2.5% and 5% XFZYD-CS for 48 h. (A) HUVECs proliferation assessed by MTT assay. (B) Images of HUVECs after treatment with XFZYD-CS for 48 h, and (C) migration of HUVECs determined by Boyden chamber migration assay. (D) HUVECs adhesion determined by gelatin adhesion assay. Values were means ± S.D. (n=6). *P<0.05 compared with B-S treatment group.
XFZYD facilitates the migration of HUVECs
To study the effect of XFZYD on HUVECs migration, we used Boyden chamber assay. The data showed that 2.5% and 5% XFZYD-CS increased HUVECs migration, whereas 1.25% XFZYD-CS had no effect on HUVECs migration (Figures 1B and 1C). The result indicates that XFZYD facilitates the migration of HUVECs.
XFZYD enhances the adhesion of HUVECs
To investigate whether XFZYD induces adhesion of HUVECs, we performed gelatin adhesion assay. The data showed that 2.5% and 5% XFZYD-CS increased HUVECs adhesion, whereas 1.25% XFZYD-CS had no effect on HUVECs adhesion (Figure 1D). The result suggests that XFZYD enhances the adhesion of HUVECs.
XFZYD increases VEGF expression in HUVECs
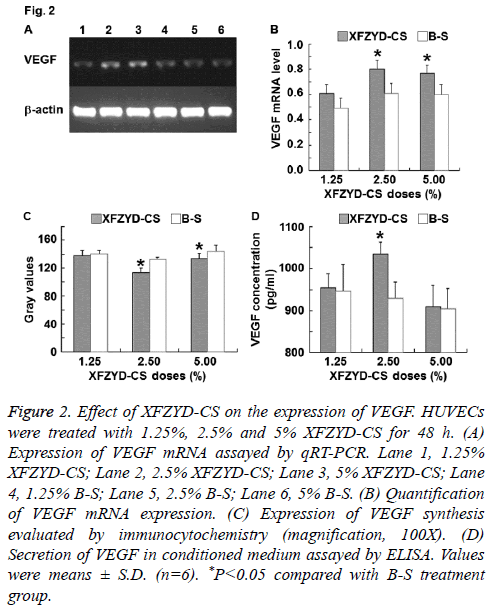
To investigate the molecule that is correlated with HUVECs proliferation, migration and adhesion, VEGF expression was measured. The data showed that 1.25% XFZYD-CS failed to up-regulate VEGF expression in HUVECs, 2.5% XFZYD-CS up-regulated VEGF expression in HUVECs, while 5% XFZYD-CS up-regulated VEGF mRNA expression and synthesis in HUVECs, but failed to induce VEGF secretion by HUVECs (Figure 2). The results indicate that XFZYD increases VEGF expression in HUVECs.
Figure 2: Effect of XFZYD-CS on the expression of VEGF. HUVECs were treated with 1.25%, 2.5% and 5% XFZYD-CS for 48 h. (A) Expression of VEGF mRNA assayed by qRT-PCR. Lane 1, 1.25% XFZYD-CS; Lane 2, 2.5% XFZYD-CS; Lane 3, 5% XFZYD-CS; Lane 4, 1.25% B-S; Lane 5, 2.5% B-S; Lane 6, 5% B-S. (B) Quantification of VEGF mRNA expression. (C) Expression of VEGF synthesis evaluated by immunocytochemistry (magnification, 100X). (D) Secretion of VEGF in conditioned medium assayed by ELISA. Values were means ± S.D. (n=6). *P<0.05 compared with B-S treatment group.
XFZYD up-regulates VEGFR2 expression in HUVECs
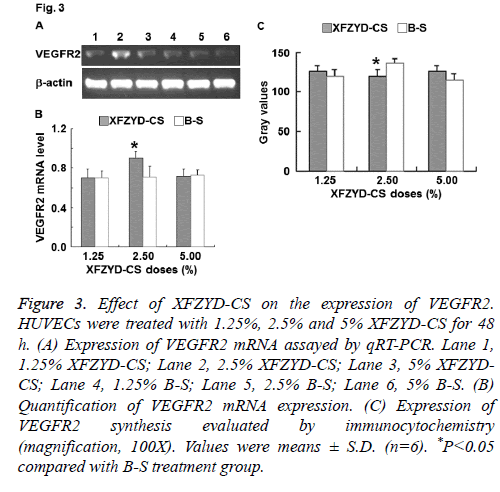
To determine the expression of VEGFR2, qRT-PCR and immunocytochemistry were employed. The data showed that only 2.5% XFZYD-CS up-regulated VEGFR2 expression in HUVECs (Figure 3). The result suggests that XFZYD upregulates VEGFR2 expression in HUVECs.
Figure 3: Effect of XFZYD-CS on the expression of VEGFR2. HUVECs were treated with 1.25%, 2.5% and 5% XFZYD-CS for 48 h. (A) Expression of VEGFR2 mRNA assayed by qRT-PCR. Lane 1, 1.25% XFZYD-CS; Lane 2, 2.5% XFZYD-CS; Lane 3, 5% XFZYDCS; Lane 4, 1.25% B-S; Lane 5, 2.5% B-S; Lane 6, 5% B-S. (B) Quantification of VEGFR2 mRNA expression. (C) Expression of VEGFR2 synthesis evaluated by immunocytochemistry (magnification, 100X). Values were means ± S.D. (n=6). *P<0.05 compared with B-S treatment group.
Discussion
XFZYD has been successfully used for the treatment of ischemia diseases by stimulating angiogenesis [12,13]. However, the cellular and molecular mechanisms underlying these pro-angiogenic effects are unclear. In the present study, we have demonstrated that XFZYD facilitates HUVECs proliferation, migration and adhesion via VEGF-VEGFR2 pathway.
Angiogenesis, the formation of new capillary blood vessels, plays an important role in the development of ischemic diseases [21]. Endothelial cells proliferation, migration and adhesion are key steps in angiogenesis [22]. Here, we have investigated the effects of XFZYD on these activities using MTT assay, Boyden chamber migration assay and gelatin adhesion assay, respectively. The results have shown that 1.25% XFZYD-CS has no effect on these activities, 2.5% XFZYD-CS promotes all these activities, while 5% XFZYDCS only enhances the migration and adhesion of HUVECs. These data suggest that XFZYD induces the proliferation, migration and adhesion of endothelial cells for angiogenesis. It speculated that XFZYD does not non-selectively stimulate angiogenesis, but may primarily maintain vessel growth under physiological or repairing conditions, and have a different activity in atheromatous plaque [14].
Numerous molecules have been reported to take part in the process of angiogenesis. Of note, VEGF-VEGFR2 pathway plays important roles in the proliferation, migration and adhesion of HUVECs. In the present study, 1.25% XFZYD-CS has no effect on VEGF and VEGFR2 expression, 2.5% XFZYD-CS increases the expression of VEGF and VEGFR2, and 5% XFZYD-CS only stimulates intracellular VEGF expression. These results suggest that 2.5% XFZYD-CS induces the proliferation, migration and adhesion of HUVECs by stimulating intracellular VEGF expression and VEGF secretion, and promoting VEGF binding to VEGFR2. In addition, 5% XFZYD-CS enhances migration and adhesion of HUVECs by inducing intracellular VEGF expression. However, 5% XFZYD-CS fails to induce HUVECs proliferation, suggesting that 5% XFZYD-CS does not promote the secretion of VEGF or the expression of VEGFR2. The mechanism to promote angiogenesis is very complexed. It not only includes up-regulating all relevant angiogenic factors, but also includes down-regulating all the relevant inhibitory factors. This phenomenon is also manifested in chemokine superfamily, in which different members have different effects on angiogenesis.
It is reported that XFZYD-CS induces ECV304 cell angiogenesis by causing significant cellular changes at 48 h and genetic changes at as early as 24 h according to microarray analysis [23]. In addition, TGFβ2 and VEGFC are upregulated, CXCL6, CXCL10, CDH5 and EGF are downregulated, and VEGF-VEGFR2 is not altered [23]. This may be due to the difference between cell lines.
Angiogenesis is regulated by a series of pro- and antiangiogenic molecules. The results suggest that XFZYD promotes proliferation, migration and adhesion of endothelial cells by up-regulating VEGF-VEGFR2 pathway. However, it remains to be further investigated whether this pathway is the sole pathway that modulates XFZYD-induced endothelial cell proliferation, migration and adhesion. In conclusion, the present study demonstrates that XFZYD induces the proliferation, migration and adhesion of HUVECs through upregulating VEGF-VEGFR2 pathway. The intriguing findings in vivo provide cellular and molecular basis for understanding the mechanisms of XFZYD in the treatment of ischemic disease. The study also provides a basis for further researches on angiogenesis mechanism.
Acknowledgements
This work was supported by the Jingzhou Central Hospital of Cardiology, The Second Clinical Medical College, Yangtze University.
Disclosures
All authors declare no financial competing interests. All authors declare no non-financial competing interests.
References
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature 2005; 438: 932-936.
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996; 86: 353-364.
- Carmeliet P. Angiogenesis in health and disease. Nat Med 2003; 9: 653-660.
- Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part I: angiogenic cytokines. Circulation 2004; 109: 2487-2491.
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocrine Rev 2004; 25: 581-611.
- Ferrara N, Gerber HP, Lecouter J. The biology of VEGF and its receptors. Nat Med 2003; 9: 669-676.
- Rahimi N. Vascular endothelial growth factor receptors: molecular mechanisms of activation and therapeutic potentials. Exp Eye Res 2006; 83: 1005-1016.
- Otrock ZK, Mahfouz RA, Makarem JA, Shamseddine AI. Understanding the biology of angiogenesis: review of the most important molecular mechanisms. Blood Cells Mol Dis 2007; 39: 212-220.
- Abedi H, Zachary I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J BiolChem 1997; 272: 15442-15451.
- Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res 2005; 65: 550-563.
- Zachary I, Gliki G. Signalling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res 2001; 49: 568-581.
- Gao D, Song J, Hu J, Lin J, Zheng L, Cai J, Du J, Chen K. Angiogenesis promoting effects of Chinese herbal medicine for activating blood circulation to remove stasis on chick embryo chorio-allantoic membrane. ZhongguoZhong Xi Yi Jie He ZaZhi 2005; 25: 912-915.
- Gao D, Lin W, ZhengLp, Chen XZ, Song J, Chen KJ. Effect of Xuefuzhuyu decoction on endothelial progenitor cells migrating from rat marrow. Zhobng Xi Yi Jie He Xin NaoXue Guan Bing ZaZhi 2007; 5: 829-831.
- Song J, Chen WY, Wu LY, Zheng LP, Lin W, Gao D, Kaptchuk TJ, Chen KJ. A microarray analysis of angiogenesis modulation effect of Xuefu Zhuyu decoction on endothelial cells. Chin J Integr Med 2012; 18: 502-506.
- Gao D, Chen Wy, Wu L, Zheng L, Lin W, Lu B, Song J, Chen K. Effect of Xuefu Zhuyu decoction on migration of endothelial cells. Chin J ExpTrad Med Formul 2011; 17; 120-124.
- Sun J, Bi Y, Guo L, Qi X, Zhang J, Li G, Tian G, Ren F, Li Z. BuyangHuanwu decoction promotes growth and differentiation of neural progenitor cells: using a serum pharmacological method. J Ethnopharmacol 2007; 113: 199-203.
- Jin X, Shen G, Gao F, Zheng X, Xu X, Shen F, Li G, Gong J, Wen L, Yang X, Bie X. Traditional Chinese drug ShuXueTong facilitates angiogenesis during wound healing following traumatic brain injury. J Ethnopharmacol 2008; 117: 473-477.
- Shen J, Dicorleto PE. ADP stimulates human endothelial cell migration via P2Y1 nucleotide receptor-mediated mitogen-activated protein kinase pathways. Circ Res 2008; 102: 448-456.
- Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res 2007; 76: 29-40.
- He MF, Liu L, Ge W, Shaw PC, Jiang R. Antiangiogenic activity of Tripterygiumwilfordii and its terpenoids. J Ethnopharmacol 2009; 121: 61-68.
- Pandya NM, Dhalla NS, Santani DD. Angiogenesis--a new target for future therapy. VasculPharmacol 2006; 44: 265-274.
- Fan TP, Yeh JC, Leung KW, Yue PY, Wong RN. Angiogenesis: from plants to blood vessels. Trends PharmacolSci 2006; 27: 297-309.