Research Article - Biomedical Research (2017) Volume 28, Issue 14
Weight loss predicts poor prognosis in patients treated with concurrent chemoradiotherapy for stage III non-small cell lung cancer
1Department of Oncology, Zhongnan Hospital of Wuhan University, Wuhan, Hubei, PR China
2Departments of Oncology, the Seventh Affiliated Hospital, Sun Yat-sen, University, Shenzhen, Guangdong, PR China
- *Corresponding Author:
- Conghua Xie
Department of Oncology
Zhongnan Hospital of Wuhan University, PR China
Accepted on June 16, 2017
Abstract
The aims of this study were to investigate the impact of weight loss experienced during initial Concurrent Chemoradiotherapy (C-CRT) on survival outcome of patients with stage III Non-Small Cell Lung Cancer (NSCLC). We retrospectively reviewed 98 patients with stage III NSCLC who received CCRT from January 2007 to December 2010. Patients were treated with systemic chemotherapy regimen of Cisplatin/Docetaxel and concurrent thoracic radiotherapy at a median dose of 66 Gy (range 60 to 70 Gy). Weight loss was defined as weight loss of more than 5% during C-CRT. In 60% of the patients weight loss was observed. In our retrospective clinical study, Kaplan-Meier curves show that patients with weight loss have poorer Progression-Free Survival (PFS) and Overall Survival (OS) (P=0.005, 0.001). Multivariate Cox regression analysis indicated that weight loss could influence the overall survival time of patients in stage III NSCLC, with the hazard ratio of 1.869 (95% CI, 1.175-2.975; P=0.008). Weight loss may be a useful prognostic factor to predict treatment outcome in stage III NSCLC treated with C-CRT.
Keywords
Concurrent chemoradiotherapy, Non-small cell lung cancer, Weight loss.
Introduction
Lung cancer is one of the most commonly seen malignancies in the world which accounts for 13% of all malignancies, and the Non-Small Cell Lung Cancer (NSCLC) occupies 80% of all lung cancers, among which 1/3 of patients are locally advanced stage III patients that are not candidates for surgery [1]. Furuse et al. showed that RTOG9410 and NPC9501 verified in the phase III clinical trials that Concurrent Chemoradiotherapy (C-CRT) could prolong the median survival for about 2 to 3 months compared with sequential radiochemotherapy, rendering it the standard treatment for locally advanced lung cancer [2-4]. Age, gender, smoking, Tumor Node Metastasis (TNM) staging [5], Epidermal Growth Factor Receptor (EGFR) status [6] were all prognostic factors for stage III NSCLC.
Obesity is a global epidemic with major individual and public health consequences. In large population-based studies, it also has been associated with increased risk and mortality of most cancers, including colon and rectum cancer, breast cancer, and pancreatic cancer [7-9]. But there are also reports of the opposite conclusion. A study on impact of weight loss on survival after chemoradiation for locally advanced head and neck cancer indicated that weight loss before and during chemoradiation was commonly observed. Weight loss before but not during treatment was associated with worse survival [10].
Lung cancer is a consumptive disease. Similar to head and neck cancer, there are many toxic side effects during C-CRT, and weight loss during treatment is common. Lung cancer is the No.1 cancer in China, which shows a rising trend. At present, there is little research regarding the relation between body weight changes during treatment and the prognosis for NSCLC in China. This experiment aimed to evaluate the prognostic value of body weight changes in the untreated patients with locally advanced NSCLC during C-CRT. The main study endpoint was the overall survival time.
Materials and Methods
Clinical data
The patient inclusion criteria: all patients with locally advanced NSCLC that were newly diagnosed in the Department of Oncology, Zhongnan Hospital in Wuhan City from January 2007 to December 2010 were selected, and all of them were confirmed pathologically with NSCLC through the Computed Tomography (CT)-guided needle aspiration biopsy of lung or transbronchial lung biopsy; and all the lung cancer patients were untreated before. The patient exclusion criteria: a history of other malignancies within the recent 5 y, symptom of dysphagia before treatment, a weight loss of greater than 5% before treatment, surgical patients, relapse patients, as well as NSCLC patients that did not complete the C-CRT and consolidation chemotherapy. We took the history of weight change for 3 months before hospitalization, only patients whose weight was relatively stable were included. We have tracked 136 subjects and 38 didn’t meet the inclusion criteria. This study was approved by the medical ethical committee of Zhongnan Hospital of Wuhan University.
Therapy and weight measures
The patients received C-CRT, with the chemotherapy regimen of docetaxel combined with cis-platin (21 d in a cycle, for 2 cycles), in the meantime, three-dimensional conformal radiotherapy was given (DT 60-70Gy/2Gy/30-35F), and the original regimen was consolidated for 2 cycles after completing C-CRT. All the patients had the body weights before and after C-CRT, together with complete follow-up data. All patients were performed CT on the head, neck, chest and upper abdomen, as well as bone scanning before treatment, so as to determine the baseline levels.
Follow-up
All patients were followed up until December 31, 2015 or their death. All patients had regular reexamination for once every 3 months within 2 y after the end of treatment, once every 6 months after 3-5 y, and once every year afterwards, and they could have the reexamination at any moment in the case of any discomfort during this period.
Data collection
The body weights of all patients before and after C-CRT were recorded, which were divided into the non-weight loss or weight loss of ≤ 5% group and the weight loss of >5% group with reference to the previous studies [11]. We collected the clinical data of patients from the record room, including age, sex, performance status, smoking status, T stage, N stage, as well as pathological data such as pathological pattern, pathological grade, and treatment-associated side reactions like radiation esophagitis.
Statistical analysis
SPSS20.0 statistical software was applied in analyzing all data. Chi-square test was utilized to analyse the differences in factors such as clinical data, pathological data and radiation esophagitis between patients in the two groups. Kaplan-Meier method and log-rank test method were adopted to analyse and compare the survival curve of the two groups of patients. Overall Survival (OS) was analysed from the day of diagnosis until death or the last follow-up. Progression-Free Survival (PFS) was calculated as the time that elapsed between the date of treatment and the date of relapse or progressive disease. Relative Risks (RR) of death associated with weight change and other variables were estimated using univariate and multivariate Cox proportional hazards model. The difference with the P<0.05 was deemed as statistically significant.
Results
Characteristics of the objects
Altogether 98 patients were enrolled in this research, including 50 male and 48 female, with 40 patients in the non-weight loss or weight loss of ≤ 5% group and 58 in the weight loss of >5% group. Age of patients ranged from 36 to 75 y of age, with median age of 60. According to UICC TNM staging criteria in 2002, 64 patients were stage III A and 34 III B, 23 for squamous cell carcinoma, 68 for adenocarcinoma, 7 for other histology classification. There were no statistical differences in aspects like clinical data and tumor between the two groups of patients (P>0.05), but there was difference in the occurrence rate of radiation esophagitis between the two groups (P=0.044), the results of which were shown in Table 1.
| Patient characteristics | Weight change | P value | |
|---|---|---|---|
| Non-weight loss or weight loss ≤ 5% | Weight loss>5% | ||
| Age (y) | 0.369 | ||
| ≤ 60 | 17 | 30 | |
| >60 | 23 | 28 | |
| Sex | 0.14 | ||
| Male | 24 | 26 | |
| Female | 16 | 32 | |
| ECOG PS | 0.224 | ||
| 0 | 32 | 40 | |
| 1, 2 | 8 | 18 | |
| T classification | 0.434 | ||
| T1+T2 | 11 | 12 | |
| T3+T4 | 29 | 46 | |
| N classification | 0.819 | ||
| N0+N1 | 17 | 26 | |
| N2+N3 | 23 | 32 | |
| Histology classification | 0.777 | ||
| Squamous carcinoma | 10 | 13 | |
| Adenocarcinoma | 28 | 40 | |
| Others | 2 | 5 | |
| Smoking | 0.635 | ||
| No | 16 | 26 | |
| Yes | 24 | 32 | |
| G classification | 0.54 | ||
| G1 | 11 | 18 | |
| G2 | 16 | 17 | |
| G3 | 13 | 23 | |
| Radiation esophagitis | 0.044 | ||
| G1-2 | 33 | 37 | |
| G3-4 | 7 | 21 | |
Table 1. Correlation between weight change and clinical characteristics in stage III NSCLC.
Survival of the objects
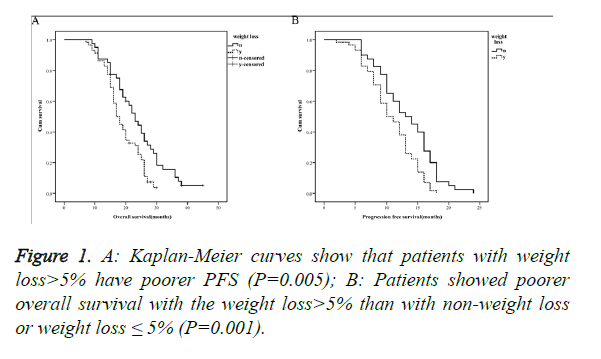
The 2-year overall survival of the non-weight loss or weight loss of ≤ 5% group was 42.5% (17 of 40), with the median survival time of 23 months, while those in the weight loss of >5% group were 27.6% (16 of 58) and 17 months, respectively, with the differences between the two groups being of statistical significance (P=0.005, Figure 1A). The median progression free survival time of the non-weight loss or weight loss of ≤ 5% group and the weight loss of >5% group were 13 and 10 months, respectively, with the differences being of statistical significance (P=0.001, Figure 1B).
Univariate and multivariate analyses
In the univariate Cox regression analyses, bad performance status, smoking and weight loss could influence the overall survival time of patients, with the hazard ratio of 1.662 (95% CI, 1.039-2.657; P=0.034), 1.548 (95% CI, 1.015-2.361; P=0.043), and 1.855 (95% CI, 1.179-2.919; P=0.008), respectively. Age, sex, T-stage, N-stage, pathological pattern, pathological grade and radiation esophagitis showed no correlation with the overall survival time of patients. The results were shown in Table 2.
| Patient characteristics | RR (95% CI) | P value |
|---|---|---|
| Age (y) | ||
| ≤ 60 | 1 | |
| >60 | 0.744 (0.492-1.124) | 0.16 |
| Sex | ||
| Male | 1 | |
| Female | 1.035 (0.685-1.566) | 0.869 |
| ECOG PS | ||
| 0 | 1 | |
| 1, 2 | 1.662 (1.039-2.657) | 0.034 |
| T classification | ||
| T1+T2 | 1 | |
| T3+T4 | 0.713 (0.438-1.160) | 0.173 |
| N classification | ||
| N0+N1 | 1 | |
| N2+N3 | 0.955 (0.631-1.447) | 0.83 |
| Histology classification | ||
| Squamous carcinoma | 1 | |
| Adenocarcinoma | 1.016 (0.615-1.678) | 0.95 |
| Smoking | ||
| No | 1 | |
| Yes | 1.548 (1.015-2.361) | 0.043 |
| G classification | ||
| G1 | 1 | |
| G2 | 1.179 (0.703-1.979) | 0.533 |
| G3 | 1.234 (0.743-2.048) | 0.381 |
| Radiation esophagitis | ||
| G1-2 | 1 | |
| G3-4 | 1.401 (0.874-2.247) | 0.161 |
| Weight change | ||
| Non-weight loss or weight loss ≤ 5% | 1 | |
| Weight loss>5% | 1.855 (1.179-2.919) | 0.008 |
Table 2. Univariate Cox regression analyses of potential prognostic for stage III NSCLC.
Multivariate Cox regression analysis indicated that weight loss could influence the overall survival time of patients, with the hazard ratio of 1.869 (95% CI, 1.175-2.975; P=0.008), and it was independent of age, sex, T-stage, N-stage, pathological pattern, pathological grade, smoking status, performance status and radiation esophagitis. The results were shown in Table 3.
| Patient characteristics | RR (95% CI) | P value |
|---|---|---|
| ECOG PS | ||
| 0 | 1 | |
| 1, 2 | 1.433 (0.888-2.312) | 0.141 |
| Smoking | ||
| No | 1 | |
| Yes | 1.614 (1.052-2.476) | 0.028 |
| Weight change | ||
| Non-weight loss or weight loss ≤ 5% | 1 | |
| Weight loss>5% | 1.869 (1.175-2.975) | 0.008 |
Table 3. Multivariate Cox regression analysis of potential prognostic factors for stage III NSCLC.
Discussion
Primary bronchogenic carcinoma is referred to as lung cancer for short, and it is one of the malignancies with the highest morbidity and mortality in the world. According to the World Health Organization (WHO), an annual of over 1.2 million new lung cancer cases occur in the world, and an annual of 1.1 million cases die of lung cancer, which shows an ascending trend year by year, ranking the top among malignancies [1]. Lung cancer is a kind of severe consumptive disease, with high occurrence rate of malnutrition among lung cancer patients. Some degree of weight loss has been noted in 54% to 80% of NSCLC patients as an early event upon diagnosis [12], which negatively alters patients’ QOL, response to treatment, and prognosis [13].
Radiotherapy and chemotherapy are the common methods for treating malignancies in clinic. However, radiochemotherapy will have severe influence on the systemic conditions of patients in the meantime of killing tumor cells, it can directly inhibit cell proliferation and differentiation, and induce cell apoptosis and mitochondrial function disorder, which will not only result in various degrees of nutritional risks in patients receiving radiochemotherapy, but also cause damage to the body function. Relevant research demonstrates that malnutrition is the extremely common complication of radiochemotherapy, with 50% to 80% of the patient receiving radiochemotherapy having various degrees of malnutrition [14]. In this research, the patients with the weight loss of greater than 5% account for 60% of the total population, which is consistent with it. Unsal et al. utilized the subjective test method to evaluate the nutritional status of patients receiving radiochemotherapy, and the research results found that at the beginning of radiotherapy, the occurrence rate of malnutrition in patients was 31%, which increased to 43% at the end of radiotherapy [15].
Currently, there are many methods to evaluate the nutritional status of tumor patients, and the most commonly used one is body weight measurement, which is simple and convenient, economic and non-invasive, and can be used to preliminarily evaluate the nutritional status of patients. The standard treatment for patients with locally advanced NSCLC is C-CRT, but it is associated with high occurrence rate of radiation esophagitis as a result of its specificness of treatment [16]. In this research, among the patients with the weight loss of >5%, the occurrence rate of grade 2 and above radiation esophagitis is higher than that in patients with no obvious weight loss. Esophagitis will affect patient eating and aggravate malnutrition that can delay the healing of esophagus mucosa, rendering further aggravated esophagitis. In this research, we apply Kaplan-Meier method and log-rank test method and discover that there are marked differences in PFS and OS between patients with weight loss and those with no weight loss. In addition, univariate and multivariate Cox regression analyses indicates that weight loss is a risk factor for the overall survival, which is also of statistical significance, and it is an independent prognostic indicator for the untreated NSCLC patients receiving radiochemotherapy. Radiation esophagitis has no correlation with the overall survival time, and some patients develop severe esophagitis but show no obvious weight loss. Therefore, the author considers that radiation esophagitis will result in weight loss in patients, but it is not the only reason for weight loss. Some scholars observe that patients develop certain proportion of weight loss in the early stage of C-CRT for locally advanced NSCLC, namely, before the occurrence of radiation esophagitis, and the weight loss also brings poor prognosis [17,18].
Relevant research indicates that the reasons responsible for the weight loss in patients with locally advanced lung cancer during the concurrent radiochemotherapy mainly include the following: malnutrition induced by the disease itself, as a kind of rapidly growing cell, tumor requires a large amount of raw materials and energy for its proliferation, thus competitively inhibiting the growth and metabolism of the normal tissues in the body [19]. In addition, the tumor produces and releases more biological factors, such as inflammatory cytokine. The systemic inflammatory response may be related to weight loss, increased basal metabolic rate, enhanced lipolysis and reduced body function of tumor patients [20]. During the radiotherapy, nausea, vomiting, and loss of appetite are the most common gastrointestinal adverse reaction, especially for patients who receive concurrent radiochemotherapy [21].
Furthermore, lung cancer patients have great mental stress, and 5% to 44% of patients have negative emotions like depression, distress, sadness, tension, anxiety, uneasiness and fearfulness in the first clear diagnosis [22]. The disease-induced mental stress may produce corresponding negative psychological reactions, which directly influence the intake of nutrient in patients, giving rise to decreased nutritional status and therapeutic effects of patients [23]. Patients with malnutrition are frequently accompanied with anemia, hypoproteinemia and weakened immunity; in some severe cases, the patients are forced to suspend the treatment, and the reduced body immune function, anti-cancer ability and tolerance to treatment result in lowered therapeutic effects and increased complications in patients, which may be the major reason that influences the survival and distant metastasis free survival.
A previous study in 425 stage 3B NSCLC patients has demonstrated that weight loss (BMI reduction>0.5 kg/m2) during C-CRT is strongly associated with inferior survival outcomes compared to weight preservation/gain [24]. Approximately, weight gain during treatment may bring excellent prognosis. There was a retrospective analysis of 92 patients treated with definitive split-course CRT between 2004 and 2010 at Rush University Medical Center. Patients who experienced weight gain were significantly more likely to survive (3 y OS, 55% vs. 31%; P=0.04) and prolonged DMFS (Distant Metastasis-Free Survival) resulted. Weight gain was the only significant predictor of survival on multivariate analysis [25].
Numerous prognostic factors of the locally advanced NSCLC have been discovered at present, such as age, sex, smoking status, performance status, T-stage and lymph node status, and all these factors are included in our research. However, this experiment is a retrospective study with relatively small sample size; therefore, prospective studies with large sample size and multi-population are required in the future to probe into the relation between body weight changes and the prognosis for locally advanced NSCLC.
Combination of nutrition support can partly improve the nutritional status, immunity and quality of life of patients, and improve the completion rate and therapeutic effects of radiochemotherapy. In addition, it can play a psychological comfort role to a great extent, and improve the state of mind of patients, thus enhancing the quality of life of lung cancer patients during and after chemotherapy. To sum up, the occurrence rate of weight loss in patients with non-small cell lung cancer during concurrent radiochemotherapy is high, which is also one of the independent adverse factors that influence the prognosis. We need to pay more attention to the weight loss during C-CRT. Furthermore, we suggest aggressive nutritional and psychological monitoring, evaluation and intervention from start of C-CRT. It may be possible to change the prognosis of these patients.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5-29.
- Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, Katagami N, Ariyoshi Y. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 1999; 17: 2692-2699.
- Curran WJ, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, Movsas B, Wasserman T, Rosenthal SA, Gore E, Machtay M, Sause W, Cox JD. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011; 103: 1452-1460.
- Fournel P, Robinet G, Thomas P, Souquet PJ, Lena H, Vergnenegre A, Delhoume JY, Le Treut J, Silvani JA, Dansin E, Bozonnat MC, Daures JP, Mornex F, Perol M, Groupe Lyon-Saint-Etienne dOncologie Thoracique-Groupe Français de Pneumo-Cancérologie. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne dOncologie Thoracique-Groupe Francais de Pneumo-Cancérologie NPC 95-01Study. J Clin Oncol 2005; 23: 5910-5917.
- Wang WP, Yan XL, Li WM, Ni YF, Zhao JB, Lu Q, Wang XJ, Sun Y, Chen P, Yan BY, Cui Y, Zhang ZP, Li XF. Clinicopathologic features and prognostic implications of Gankyrin protein expression in non-small cell lung cancer. Pathol Res Pract 2015; 211: 939-947.
- Park DI, Kim SY, Kim JO, Jung SS, Park HS, Moon JY, Chung CU, Kim SS, Seo JH, Lee JE. The prognostic value of the tumor shrinkage rate for progression-free survival in patients with non-small cell lung cancer receiving gefitinib. Tuberc Respir Dis (Seoul) 2015; 78: 315-320.
- Morrison DS, Parr CL, Lam TH, Ueshima H, Kim HC, Jee SH, Murakami Y, Giles G, Fang X, Barzi F, Batty GD, Huxley RR, Woodward M. Behavioural and metabolic risk factors for mortality from colon and rectum cancer: analysis of data from the Asia-Pacific Cohort Studies Collaboration. Asian Pac J Cancer Prev 2013; 14: 1083-1087.
- Kaviani A, Neishaboury M, Mohammadzadeh N, Ansari-Damavandi M, Jamei K. Effects of obesity on presentation of breast cancer, lymph node metastasis and patient survival: a retrospective review. Asian Pac J Cancer Prev 2013; 14: 2225-2229.
- Pelucchi C, Galeone C, Polesel J, Manzari M, Zucchetto A, Talamini R, Franceschi S, Negri E, La Vecchia C. Smoking and body mass index and survival in pancreatic cancer patients. Pancreas 2014; 43: 47-52.
- Ghadjar P, Hayoz S, Zimmermann F, Bodis S, Kaul D, Badakhshi H, Bernier J, Studer G, Plasswilm L, Budach V, Aebersold DM, Swiss Group for Clinical Cancer Research (SAKK). Impact of weight loss on survival after chemoradiation for locally advanced head and neck cancer: secondary results of a randomized phase III trial (SAKK 10/94). Radiat Oncol 2015; 10: 21.
- van der Meij BS, Phernambucq EC, Fieten GM, Smit EF, Paul MA, van Leeuwen PA, Oosterhuis JW. Nutrition during trimodality treatment in stage III non-small cell lung cancer: not only important for underweight patients. J Thorac Oncol 2011; 6: 1563-1568.
- Tan BH, Fearon KC. Cachexia: prevalence and impact in medicine. Curr Opin Clin Nutr Metab Care 2008; 11: 400-407.
- Ross PJ, Ashley S, Norton A, Priest K, Waters JS, Eisen T, Smith IE, Obrien ME. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer 2004; 90: 1905-1911.
- Ramnath N, Dilling TJ, Harris LJ, Kim AW, Michaud GC, Balekian AA, Diekemper R, Detterbeck FC, Arenberg DA. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer. American College of Chest Physicians evidence-based clinical practice guidelines (3rd edn.). Chest 2013; 143: 314-340.
- Unsal D, Mentes B, Akmansu M, Uner A, Oguz M, Pak Y. Evaluation of nutritional status in cancer patients receiving radiotherapy: a prospective study. Am J Clin Oncol 2006; 29: 183-188.
- Bar-ad V, Leiby B, Witek M, Xiao Y, Cui Y, Dai Y, Cao J, Axelrod R, Campling B, Both S, Werner-Wasik M. Treatment-related acute esophagitis for patients with locoregionally advanced non-small cell lung cancer treated with involved-field radiotherapy and concurrent chemotherapy. Am J Clin Oncol 2014; 37: 433-437.
- Op den Kamp CM, De Ruysscher DK, van den Heuvel M, Elferink M, Houben RM, Oberije CJ, Bootsma GP, Geraedts WH, Pitz CC, Langen RC, Wouters EF, Schols AM, Dingemans AM. Early body weight loss during concurrent chemo-radiotherapy for non-small cell lung cancer. J Cachexia Sarcopenia Muscle 2014; 5: 127-137.
- Sanders KJ, Hendriks LE, Troost EG, Bootsma GP, Houben RM, Schols AM, Dingemans AM. Early Weight Loss during Chemoradiotherapy Has a Detrimental Impact on Outcome in NSCLC. J Thorac Oncol 2016; 11: 873-879.
- Skipworth RJ, Moses AG, Sangster K, Sturgeon CM, Voss AC, Fallon MT, Anderson RA, Ross JA, Fearon KC. Interaction of gonadal status with systemic inflammation and opioid use in determining nutritional status and prognosis in advanced pancreatic cancer. Support Care Cancer 2011; 19: 391-401.
- McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 2009; 12: 223-226.
- Feyer PC, Maranzano E, Molassiotis A, Roila F, Clark-Snow RA, Jordan K. Radiotherapy-induced nausea and vomiting (RINV): MASCC/ESMO guideline for antiemetics in radiotherapy: update 2009. Support Care Cancer 2011; 19: 5-14.
- Turner NJ, Muers MF, Haward RA, Mulley GP. Psychological distress and concerns of elderly patients treated with palliative radiotherapy for lung cancer. Psychooncology 2007; 16: 707-713.
- Tian J, Chen ZC, Hang LF. Effects of nutritional and psychological status in gastrointestinal cancer patients on tolerance of treatment. World J Gastroenterol 2007; 13: 4136-4140.
- Topkan E, Parlak C, Selek U. Impact of weight change during the course of concurrent chemoradiation therapy on outcomes in stage IIIB non-small cell lung cancer patients: retrospective analysis of 425 patients. Int J Radiat Oncol Biol Phys 2013; 87: 697-704.
- Sher DJ, Gielda BT, Liptay MJ, Warren WH, Batus M, Fidler MJ, Garg S, Bonomi P. Prognostic significance of weight gain during definitive chemoradiotherapy for locally advanced non-small-cell lung cancer. Clin Lung Cancer 2013; 14: 370-375.