- Biomedical Research (2015) Volume 26, Issue 3
Volatile constituents in Du Jiao Lian and their effects on proliferation of breast cancer T47D cell lines.
Tao Jiang1,2, Jiefeng Zhang3, Yongqing Zhang3, Yongteng Shi2, Jian Wang2, Rong Ma1*
1Department of Breast Surgery, Qilu Hospital of Shandong University, Jinan 250012, China
2Department of General Surgery, Qingzhou People’s Hospital, Qingzhou, Shandong 262500, China
3Department of General Surgery, Weifang People’s Hospital, Weifang, Shandong 261500, China
- Corresponding Author:
- Rong Ma
Department of Breast Surgery
Qilu Hospital of Shandong University
Jinan 250012
China
Accepted date: March 27 2015
Abstract
To identify volatile constituents in Typhonium giganteum Engl., and clarify their effects on proliferation of breast cancer T47D cell lines. Volatile constituents in Typhonium giganteum Engl. are extracted by steam distillation, and determined by GC-MS. 24, 48 and 72 h after treatment of T47D cells with different concentrations of Typhonium giganteum Engl. volatile (DJL-V) constituents, methyl thiazolyl tetrazolium (MTT) colorimetric assay, inverted optical microscopic observation, flow cytometry (FCM) and agarose gel electrophoresis are used to detect cell proliferation and apoptosis. 15 major chemical constituents are isolated and identified from DJL-V, with a detection rate of 76.41%. DJL-V can significantly inhibit T47D cell proliferation in a time- and concentration-dependent manner within a certain concentration range, and can induce its apoptosis. T47D cell apoptosis rates are 7.51%, 13.45% and 13.79%, respectively, 24, 48 and 72 h after treatment with 100 g/L DJL-V. The degree of apoptosis is positively correlated with the time. 72 h after treatment, distribution of S phase (DNA synthesis phase) cells increases significantly. DJL-V has proliferation inhibitory and apoptosis inducing effects on T47D cells; and its anti-tumor mechanism is associated with the induction of apoptosis.
Keywords
Typhonium giganteumEngl.; Volatile oil; GC-MS; Breast cancer; T47D
Introduction
Typhonium giganteum Engl. is a plant in the genus Typhonium of the family Araceae, whose main medicinal part is dried tuber. In the 2010 edition of the "Pharmacopoeia of the People's Republic of China", its name is recorded as Bai Fu Zi, which is a common traditional Chinese medicine. The plant is distributed in most parts of China, mainly in places such as Henan, Gansu and Hubei. Typhonium giganteum Engl. is acrid, sweet and warm, which enters the stomach and liver meridians, and has detoxifying and mass-dissipating actions. It is used for stroke, phlegm obstruction, facial paralysis, unilateral or bilateral headache, rheumatism, pyretic arthralgia, tetanus, tumors and immune regulation[1-3]. Modern studies have found that Typhonium giganteum Engl. has curative effects on snakebites, scrofula and traumatic injuries[4-5]. In this paper, volatile oil is extracted from dried tubers of Typhonium giganteum Engl. by steam distillation, and analyzed by GC-MS technique. 15 chemical constituents are analyzed and identified.
Global incidence of breast cancer is rather high, which has shown a growing trend in recent years[6-8]. Use of chemotherapy drugs is one of the primary treatments of breast cancer, but existing chemotherapy drugs all have their respective shortcomings. So the search for effective novel anticancer drugs with small toxic side effects is of important practical significance. In TCM clinical practice, Typhonium giganteum Engl. has been widely used in the treatment of various malignant diseases[9-10], and has exhibited good curative effects. Treatment of breast cancer with Typhonium giganteum Engl. volatile oil (DJL-V) has never been reported at home and abroad. In this study, a variety of detection methods are used to observe the effects of DJL-V on proliferation, apoptosis and cell cycle of breast cancer T47D cells, and explore the basis for treatment of breast cancer by DJL-V.
Instruments and materials
Instruments
HP-5988A GC-MS system, Hewlett Packard, USA; OV- 17 fused silica capillary column L25m×0.20mm; SMYT-832 ultrafine pulverizer (Yutong Machine Co., Ltd., Shenyang). CO2 incubator (Shanghai Instrument Co., Ltd.), clean bench (Yijing Co., Ltd., Jiangsu), microplate reader (Biocell), inverted optical microscope (Olympus, Japan), tabletop centrifuge (Shanghai Scien tific Instrument Factory), flow cytometer (Coulter EPICSXL) and electrophoresis system (Tianqi Biotechnology Co., Ltd., Shanghai).
Reagents and materials
N-hexane and anhydrous sodium sulfate were analytical grade reagents (purchased from Kaimei Reagent Co., Ltd.). Typhonium giganteum Engl. medicinal material was purchased from Anguo Pharmaceutical Co., Ltd. in Hebei, which was identified by Professor Zhu Liping of the Nanjing Pharmaceutical University as the dried tuber of Typhonium giganteum Engl. in the family Araceae. RPMI- 1640 medium (Gibco); fetal bovine serum (Shenyang Bioengineering Technology Co., Ltd.); DMSO (Tianjin Kemiou Chemical Reagent Co., Ltd.); MTT (Fluka, USA).
Human breast cancer T47D cell lines were provided by the First Affiliated Hospital of China Medical University.
Experimental Methods
Preparation of sample solution
Dried superfine Typhonium giganteum Engl. tuber powder was passed through a 200-mesh sieve. Then, 50 g was taken, added with 2000 mL of distilled water and 10 mL of n-hexane, shaken well, and steam distilled to give 2.1 mL of volatile oil, which was subsequently dried over anhydrous sodium sulfate to serve as the test sample.
Qualitative and quantitative analysis of DJL-V
1 μL of DJL-V was taken, and injected for GC-MS determination. GC conditions: column: OV-17 fused silica capillary column L25m×0.20mm; column temperature: 25oC (maintained for 1.0 min), 40°C~280 °C (3 °C /min); gasification chamber temperature: 280oC; carrier gas: high purity nitrogen, with a flow rate of 60 mL/min; injection volume: 1 μL; split ratio: 100:1.
MS conditions: carrier gas: high purity nitrogen, with a flow rate of 40 ml/min; split ratio: 40:1; EI ionization source (70 eV), scanning range: 40~500 AMU. Other conditions were the same as the GC analysis conditions.
Qualitative analysis: Nist05 standard mass spectral library and WILEY275 mass spectral library were retrieved via HP MSD ChemStation. Meanwhile, relevant mass spectral literature analysis was consulted, in order to identify the chemical constituents of volatile substances in superfine Typhonium giganteum Engl. powder.
Quantitative analysis: Relative peak area percentage of each chemical constituent was calculated by peak area normalization via HP MSD ChemStation data processing system.
Biological activity test
T47D cells were seeded in RPMI-1640 medium (containing 10% fetal bovine serum, and 100 Unit/mL penicillin and streptomycin, pH value 7.2~7.4), and cultured in a 5% CO2 incubator at 37 o°C. The cells were passaged when they grew well.
Detection of the effect of DJL-V on T47D cell proliferation by MTT assay
After growing to logarithmic phase, T47D cells were seeded in a 96-well culture plate at 1×105 cells/mL at 190 μL per well, and cultured in a 5% CO2 incubator at 37oC for 4 h. After precipitation, the cells were treated with 10 μL of three different concentrations (50, 100 and 150 g/L) of DJL-V, respectively. Control group was added with 10 μL of sterile double distilled water; in addition, a nonapoptosis group was also set up. Each group had four wells. After culturing in a 37oC, 5% CO2 incubator for 24, 48 and 72 h, respectively, 20 μL of 5 g/L MTT was added, and the incubation was continued for another 4 h. Then, the supernatant was discarded, and the remaining was dissolved by addition of 150 μL of DMSO per well, then shaken at room temperature for 10 min, followed by measurement of OD value at 490 nm using a microplate reader. Growth inhibitory rate (IR) was calculated. IR = 1 - [(OD value of treatment group - OD value of apoptosis group) / (OD value of control group - OD value of apoptosis group)] × 100%. The above experiment was done in three replications.
Optical microscopic observation of cell morphology
Changes in growth morphology of T47D cells in the treatment and control groups after treatment with 50 g/L DJL-V for 24, 48 and 72 h were observed using an inverted microscope.
Observation of apoptotic characteristics by agarose gel electrophoresis in DNA ladder band test, cells treated with 50 g/L DJL-V for 24, 48 and 72 h, as well as control cells were taken at 1×106 cells/mL, and washed with phosphate buffered saline (PBS). After separately adding 250 μL of Tris EDTA buffer (TE, pH value 7.6), 250 μL of cell lysis buffer, 10 μL of proteinase K and 8 μL of ribonuclease A (RNaseA), the solutions were mixed well and left in a 37°C water bath overnight. After extracting once with phenol: chloroform: isoamyl alcohol (v/v/v = 25:24:1), the supernatant was added with 1/10 volume of 3 mol/L sodium acetate and 2.5~3.0 fold volume of cold anhydrous ethanol to precipitate DNA. After supernatant was removed by centrifugation at 12000 r/min, the remaining was washed once with 1 mL of 75% ethanol, and dried, followed by dissolution of DNA in 50 μL of TE. 10 μL of sample buffer was electrophoresed on 1.5% agarose gel for 1~2 h, then, DNA ladder band was observed under UV light.
FCM detection of T47D cell cycle and apoptosis Cells treated with 50 g/L DJL-V for 24, 48 and 72 h, as well as control cells were taken at 1×106 cells/mL, respectively, centrifuged at 1000 r/min to remove supernatant, and resuspended in 1 mL of 4° C precooled fixative (composition of 1 L of fixative: glucose 1.1 g, NaCl 8.0 g, KCl 0.4 g, Na3PO4·12H2O 0.39 g, KH2PO4 0.15 g, MgSO4·7H2O 0.08 g, CaCl2·2H2O 0.16 g) for fixation.
After slowly adding 3 mL of -20oC precooled 95% ethanol to make the final concentration 75%, the solutions were the ice bathed for 30 min, and then stored at 4°C. FCM detection and analysis were completed with the help of the Central Laboratory of Sichuan First People's Hospital.
Data processing
Data were analyzed and processed using SPSS 11.5 software, and statistically tested by ANOVA
Results
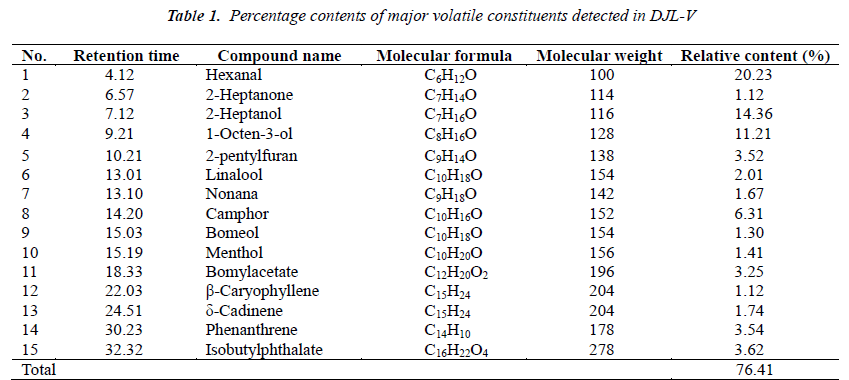
Results of volatile oil determination experiment Relative percentage contents of various chemical constituents in DJL-V are shown in Table 1.
Effect of DJL-V on T47D cell proliferation
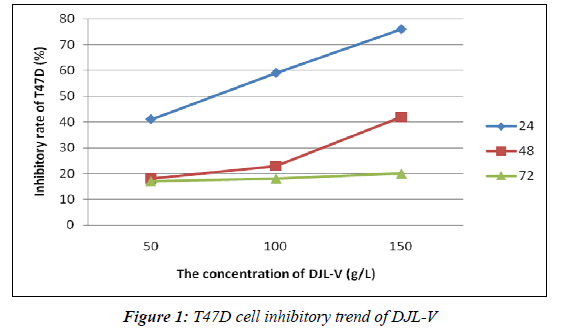
As can be from Figure 1, after treatment for 24 h, DJL-V inhibited T47D cell proliferation while having no significant inhibitory effect on normal control cells, and its optimal effective concentration was 150 g/L; when treated for 48 h, optimal effective concentration was 100 g/L; and when treated for 72 h, optimal effective concentration was 50 g/L. IR of 150 g/L DJL-V on T47D cell growth reached 41.23%, exceeding the standards for chemotherapeutic drug sensitivity (30%). It was thus clear that DJL-V had an inhibitory effect on T47D cells, with IR exhibiting marked time- and dose-dependence within a certain concentration range.
Results of optical microscopic observation of T47D cell morphology
After treating with 100 g/L DJL-V for 24, 48 and 72 h, T47D cells underwent a series of changes. Control cell were dense, morphologically intact, round and transparent. DJL-V treated cells were evidently inhibited, and cell growth was slowed. With the increase of treatment time, number of cells per unit area decreased gradually, and cell-cell junction disappeared. T47D cells had reduced transparency and brightness, smaller size, became round, and were irregularly shaped. Foreign granules appeared within the cells, nuclei were pyknotic, and cell membranes were intact but foamy.
Results of agarose gel electrophoretic observation of apoptotic characteristic ladder bands
Experimental results showed the appearance of typical DNA ladder after 1.5% agarose gel electrophoresis, indicating that the endonuclease cleaved DNA into about 200 bp fragments, thereby interfering with cell growth cycle to induce apoptosis. In addition, with increasing time, DNA fragments, ladder intensity and apoptotic cells all increased. Apoptosis showed a marked time-effect relationship.
Results of FCM detection of cell cycle and apoptosis
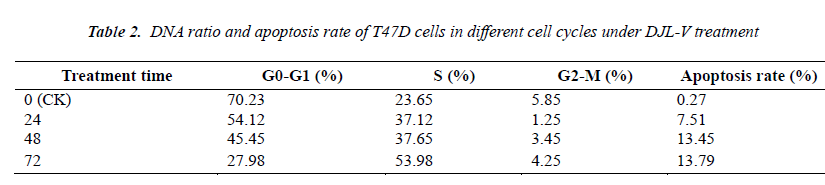
As can be seen from Table 2, the majority of control cells were in the Gap 1 phase (G1), followed by the S phase, only a minimal number of cells were in the Gap 2 phasemitosis phase (G2-M); apoptosis rate of control cells was 0.23%. After treatment of T47D cells with 100 g/L DJL-V for 24, 48 and 72 h, apoptosis rate increased with increasing treatment time, which was 8.34%, 12.1% and 12.6%, respectively, after 24, 48 and 72 h. This indicated that the degree of T47D apoptosis was positively correlated with the time.
Discussion
In this study, GC-MS method is used for extraction and analysis of volatile oil in dried Typhonium giganteum Engl. tubers, which finds 15 major chemical constituents, accounting for 76.41% of total essential oil quantity. Among them, 8 constituents have relative percentage contents higher than 3%, and 3 constituents have relative percentage contents higher than 10%, they are hexanal (20.23%), 2-heptanol (14. 36%) and 1-octen-3-ol (11.21%). It can be seen from the analysis results that aliphatic compounds make up the largest part, while aromatic compounds only account for 10%, and terpenoids occupy a higher proportion than aromatic compounds, which is about 17%. Terpenoids contained are mainly sesquiterpenes and monoterpenes. Almost all of these terpenoids have important medicinal values or are widely used in perfumes and other chemical synthetic materials; for example, the application of camphor and menthol are very common; and β-caryophyllene has a good antiinflammatory effect[11-12]. Borneol has antispasmodic, deworming and expectorant pharmacological effects[13- 14]. In addition, azulenoids generally have antibacterial, antitumor and insecticidal bioactivities[15]. This paper provides the scientific basis for profound utilization of Typhonium giganteum Engl. plant resources by extraction, analysis, identification and relative content determination of volatile constituents in Typhonium giganteum Engl.. However, the rich medicinal values of its volatile oil constituents, particularly terpenes, remain to be further studied.
Test results show that DJL-V can significantly inhibit T47D cell proliferation in a time- and concentration-dependent manner within a certain concentration range, and can induce apoptosis. Apoptosis rates of T47D cells are 7.51%, 13.45% and 13.79%, respectively, after treatment with 100 g/L DJLV for 24, 48 and 72 h; the degree of T47D apoptosis is positively correlated with the time. There is a significant increase in the distribution of S phase cells after treatment for 72 h. It can thus be seen that DJL-V has proliferation inhibitory and apoptosis inducing effects on T47D cells, and its anti-tumor mechanism is associated with the induction of apoptosis. This is consistent with the apoptosis inducing effect of Typhonium giganteum Engl. on a variety of tumor cell lines and its time- and dose-dependence reported in the literature [16]. There have also been studies suggesting that the dosedependence of apoptosis is limited to a certain range; large doses cause acute necrosis, thus weakening the apoptosis inducing effect; whereas too low doses may only cause tiny, reversible damages[3,17]. Therefore, the research of optimal dose of apoptosis inducing drugs is of important significance in clinical work.
Detection of drug sensitivity by MTT assay allows indirect understanding of subcellular states and mitochondrial changes [18], which is conducive to highly targeted individual therapy, and can improve the curative efficacy and the quality of life. This study also indirectly confirms that DJL-V has T47D mitochondrial respiration inhibitory, oxidative phosphorylation blocking and electron transfer disturbing effects, thereby leading to apoptosis. Induction of tumor cell apoptosis is the main goal and means in the research of cancer treatment[19]. Inverted microscopic observation results show that after T47D cell treatment with 100 g/L DJL-V, the number of viable cells decreased markedly whereas the number of apoptotic cells increased with increasing treatment time as compared with the control group, showing the time dependence of DJL-V's apoptosis inducing effect on T47D cells. Therefore, from the perspective of apoptosis induction, continuous small dose administration on T47D cells can improve the outcome. DJL-V can significantly induce T47D cell apoptosis, suggesting that the apoptosis induction may be one of DJL-V's anti-tumor mechanisms of action. This provides a new therapeutic strategy for future cancer treatment.
After T47D cell treatment with 100 g/L DJL-V, S phase cell distribution markedly increases with increasing time, and G2-M phase cells also somewhat increases. It can thus be seen that DJL-V can change the proliferation cycle dynamics of T47D cell lines when acting on them, which is manifested by increased S phase ratio, slightly increased G2-M phase ratio and decreased G1-S phase ratio, thereby blocking S phase and G2-M phase of tumor cells, weakening tumor cell division capacity, and inhibiting tumor proliferation. DJL-V's cell cycle arrest effect is an important cause of increased apoptotic cells. Enhancement of such an arrest effect can produce cell synchronization effect, enabling tumor cells to be distributed in the S phase, which can play a synergistic anti-tumor role together with the S phase cell cyclespecific drugs, thus providing the theoretical basis for enhancement of chemotherapeutic efficacy by combined application of DJL-V and other drugs in clinical practice. In addition, a previous study has confirmed that cells in mitotic phase are more sensitive to radiotherapy than cells in other phases[20]; so DJL-V combined with radiotherapy promises better therapeutic effect for cancer patients.
References
- Compiling Group of Traditional and Herb Medicineof China. Traditional and Herb Medicine of China.
- Shanghai: shanghai Science and Technology Publishing House 1999; 8: 530-532.
- National Pharmacopoeia Commission. Ch P 2005: 70-71.
- Li Q, Jiang C, ZuY, Song Z, Zhang B, Meng X, QiuW, Zhang L. SFE-CO2 extract from TyphoniumgiganteumEngl. tubers, induces apoptosis in human hepatoma SMMC-7721 cells involvement of a ROSmediatedmitochondrial pathway. Molecules 2011; 16: 8228-8243.
- Chen XS, Chen DH, Si JY, Tu GZ. Chemical constituents of Typhoniumgiganteum Engl. Journal of Asian Natural Products Research 2001; 3: 277-283.
- Chen XS, Wu YL, Chen DH. Structure determination and synthesis of a new cerebroside isolated from the traditional Chinese medicine Typhoniumgiganteum Engl. Tetrahedron Letters: International Organ for the Rapid Publication of Preliminary Communications in Organic Chemistry 2002; 43: 3529-3529.
- Samantha RO, David GO, Christopher J. Ormandy The mammary cellular hierarchy and breast cancer.
- Cellular and Molecular Life Sciences 2014; 71: 4301-4324.
- Joana M, Claudia G, Wilfrid R, Patrick FF, Andrea S, Rosalie J, Anouar I, Joana B, Charles D, Riccardo F. Cancer stemness in Wnt-driven mammary tumorigenesis. Carcinogenesis 2014; 35: 2-13.
- Shchurov NF. The role of tumoralstroma in prognosis of course of luminal mammarial gland cancer type A. KlinKhir 2014; 1: 35-37.
- Yin JY, Piao CJ, Yang JZ, Li J, Yang ZH, Wei YD, Zhang HG. Studie on the antitumor activity of TyphoniumgiganteumEngl. I. Journal of Changchun University of Traditional Chinese Medicine 2000; 16: 52-53.
- Wang SQ, Ni H, Wang J, Chen L. Inhibitory effects of TyphoniumgiganteumEngl. On hepatocarcinomaSMMC-7721 cells.Chinese Journal of Cell Biology 2003; 25: 185-187.
- Cheng Y, Dong Z, Liu S. β-Caryophyllene Ameliorates the Alzheimer-Like Phenotype in APP/PS1
- Mice through CB2 Receptor Activation and the PPARγPathway. Pharmacology 2014; 94: 1-12.
- Manal AA, Mutasem OT, Malek AZ, Ahmad MD. β- Caryophyllene causes regression of endometrial implants in a rat model of endometriosis without affecting fertility. European Journal of Pharmacology 2013; 702: 12-19.
- Joshi RK. Volatile composition and antimicrobial activity of the essential oil of Artemisia absinthium
- growing in Western Ghats region of North West Karnataka, India. Pharm Biol 2013; 51: 888-892.
- Liu XC, Liang Y, Shi WP, Liu QZ, Zhou L, Liu ZL. Repellent and insecticidal effects of the essential oil of Kaempferiagalanga rhizomes to Liposcelisbostrychophila(Psocoptera: Liposcelidae). Journal of
- Economic Entomology 2014; 107: 1706-1712.
- Murakami AA, Li AM, Mat-Salleh K. Screening forthe in vitro ant-i tumor-promoting activities of edible plants from Malaysia. BiosciBiotechnolBiochem2000; 64: 9-12.
- Wang SQ, Ni H, Cheng H, Wang GL, Wang TS, Chen L. Detection of differentially expressed genes
- inhepatocellularcarcinoma cells SMMC-7721 treated with Typhoniumgiganteum extract by mRNA
- differential display. ZhongguoZhong Yao ZaZhi2004; 29: 974-977.
- Xin HW. Study on chemosensitivity of 40 cases of gastric and breast cancers by MTT assay. Chinese
- Journal of Clinical Oncology 1994; 21: 699-705.
- Lv Q, Cheng Z, Yuan SL. Study on chemosensitivityof breast cancer cells by MTT assay. Sichuan Medical Journal 2001; 22: 269-274.
- Foongladda S, Roengsanthia D, Arjrattanakool W, Chuchottaworn C, Chaiprasert A, Franzblau SG.
- Rapid and simple MTT method for rifampicin and isoniazid susceptibility testing of mycobacterium tuberculosis.Int J Tuberc Lung Dis 2002; 6: 11-18.
- Xiao Y. Li JD, Shi HL, Liu JH, Feng YL, Li MD. Predictive value of in vitro MTT assay chemosensitivitytest of cytotoxic drug activity in cervical cancer.Ai Zheng 2007; 26: 386-389.