Research Article - Biomedical Research (2016) Volume 27, Issue 4
Vitamin D3 supplementation improves immune and inflammatory response in vitamin D deficient adults in Taif, Saudi Arabia
Adel Qlayel Hamdan Alkhedaide1*, Zafer Saad Alshehri2, Mohamed Mohamed Soliman1,3, Khalid Waslallah Althumali4, Helal Saad Abu-Elzahab5,6, Ahmed Abdel Aziz Baiomy6,71Department of Medical Laboratories, Faculty of Applied Medical Sciences, Taif University, Turabah, Saudi Arabia
2Department of Medical Laboratories, Faculty of Applied Medical Sciences, Shaqraa University, Aldawadmi, Saudi Arabia
3Biochemistry department, Faculty of Veterinary Medicine, Benha University, Moshtouhor, Toukh, Eygpt
4Department of General Surgery, King Faisal Medical Complex, Taif, Saudi Arabia
5Department of Biology, Faculty of Science, Taif University, Taif, Saudi Arabia
6Department of Zoology, Faculty of Science, Cairo University, Giza, Egypt
7Department of Medical Biotechnology, Faculty of Applied Medical Sciences, Taif University, Turabah, Saudi Arabia
- *Corresponding Author:
- Adel Qlayel Hamdan Alkhedaide
Department of Medical Laboratories
Faculty of Applied Medical Sciences
Taif University Turabah Saudi Arabia
Accepted on March 23, 2016
Abstract
Vitamin D deficiency is implicated in several medical conditions and has several beneficial physiological effects. The adequate daily intake of vitamin D is between 2000-4000 IU. Nowadays, vitamin D deficiency became a phenomenon among people in Taif. Therefore, to what extent vitamin D deficiency is associated with epidemics and immune system efficiency. A total of 101 (59 males) and (42 females) were recruited in this study. Sera were extracted on the first day to estimate vitamin D3, immunoglobulins, cytokines, complement proteins, rheumatoid factor and C-reactive protein as a baseline. Participants were given 4000 IU/day of vitamin D3 orally for twenty days. On the twentieth day, sera were extracted for same parameters evaluation. After twenty days of supplementation serum vitamin D3 levels increased by approximately four folds in most subjects. This increment was accompanied with a significant IgM levels increase (31% in females) and (28% in males) in which P values <0.05 corresponding to baseline, and a slight increase in IgA, C4 and C3. Inversely, serum levels of IgG significantly decreased in female subjects in which p value was 0.05< corresponding to baseline. Moreover, both interleukine-7 and tumour necrosis factor-α levels decreased for about 50% in both female and male participants. In conclusion, twenty days of 4000 IU/day of oral vitamin D3 supplementation can change vitamin D status from deficient to normal levels in individuals that assumed to be healthy. Vitamin D improves both innate and adaptive immunity and can prevent autoimmune reaction, and might serve as anti-inflammatory agent.
Keywords
Vitamin D, Immunity, Supplementation, Inflammatory, Immunoglobulins, Cytokines.
Introduction
Vitamin D is a steroid compound called 1α,25- dihydroxyvitamin D3 [1α,25(OH)2D3] [1]. Vitamin D is a life essential in mammals especially higher animals [1]. This steroid is produced in the skin in most vertebrate animals as a prohormone [2]. Cholesterol is the initial precursor of vitamin D which is converted into 7-dehydrocholesterol in large quantities in the epidermal layer [3]. 7-dehydrocholesterol in turn undergoes photolyzation by ultraviolet rays at 9,10 carbon bond in the B ring to form a pre-vitamin D3 which spontaneously isomerized to Vitamin D3 [3]. There are two major forms of Vitamin D; Vitamin D2 which called ergocalciferol and Vitamin D3 which called cholecalciferol and both are fat soluble compounds [3]. In the other context, regulating blood calcium levels is controlled by parathyroid glands via a mechanism involves vitamin D which means that there is a clear relationship between Parathyroid Hormone (PTH) and blood levels of vitamin D [4]. Many studies over the last three decades have elucidated that vitamin D deficiency is implicated in several medical conditions such as multiple sclerosis, cardiovascular mortality and cancer incidence [5,6]. Some clinical reports demonstrated that vitamin D deficiency is associated with some pathological conditions such as rheumatoid arthritis, cardiovascular disease and inflammation [7-9]. Furthermore, deficiency of vitamin D was found accompanied with autoimmune disorders in Grave’s disease, ankylosing spondylitis, systemic lupus erythematosus, and in rheumatoid arthritis patients [7-10].
Vitamin D has anti-inflammatory and immune-modulating roles and plays other several biological roles such its role in cell proliferation, cellular differentiation and bone metabolism [6-8]. Serum levels of vitamin D have been found linked with cytokine production in both monocytes and macrophages in a pathway involved MAPK phosphatase-1 upregulation [11]. Moreover, vitamin D3 reduces proliferation of monocytes and decreases the production of several growth factors and immune mediators in a study involved human annulus cells [12]. The dietary allowance of vitamin D is 200 IU and that just to prevent the incidence of osteomalacia with the full absence of sunlight exposure [13]. However, more than that dose might be required to prevent more complicated cases such as osteoporosis, hyperparathyroidism, some cancers, osteoarthritis progression, multiple sclerosis, and hypertension [13]. The daily sun light exposure might provide approximately 10000 IU [14-16]. The collected data over the last two decades revealed that the adequate daily intake of vitamin D is between 2000-4000 IU [11,13,17,18]. On the other hand, elevated serum levels of vitamin D are related to hypercalcemia, renal lesions, low serum estrogen levels and sarcoidosis [19-21]. In the kingdom of Saudi Arabia particularly in Taif, vitamin D deficiency became a phenomenon which raised several questions up and whether vitamin D deficiency underlay some common epidemics in Taif or not so, the main purpose of this study is to examine the role of vitamin D in immune system in both female and male individuals.
Materials and Methods
Experimental design and sampling
Participants were divided sexually into female (42 volunteers), male (59 volunteers). All subjects were requested to sign the consent form that designed by Medical Ethics and Research Committee (MERC) and all were aged between 30-44 years old. On the beginning day, blood sera were extracted from participants to evaluate vitamin D levels and for quantitative screening of IgM, IgG, IgA, C3, C4, IL-7, TNF-α, IL-4, CCL2, CRP and RF. Throughout the study all participants had given orally vitamin D3 (4000 IU/day) for twenty days. To assess the status of vitamin D and other biomarkers, participants provided another blood sample at the end of the course.
Materials
Vitamin D3 estimation kits were purchased from Roche Diagnostics GmbH D-68298 Mannheim Germany. Immunoglobulins, C3 and C4 complement proteins, C-reactive protein and rheumatoid factor kits were purchased from Beckman Coulter International SA,Rue Juste-Olivier 22PO Box 1059, Nyon, Switzerland. Cytokines ELISA estimation kits for TNF-alpha, Interlukin-4, Interlukin-7 and CCL2 were purchased from Biovendor R&D, Karasek 1767/1, 621 00 Brno, Czech Republic.
Instrumentation
Serum vitamin D levels were estimated using Cobas e 411, a full automated immunochemistry analyzer equipped with Electrochemiluminescence technology. Another immunochemistry system called Immage 800 was used to evaluate serum levels of immunoglobulins, C3, C4 complement proteins, C-reactive protein and rheumatoid factor
Serum extraction
Blood samples were extracted from each participant in a plain tube. Samples were centrifuged to separate serum at 3200Xg before processing.
Statistical analysis
Results are expressed as means ± S.E.M. Statistical analysis was done using ANOVA and Fischer’s post hoc test, with p<0.05 being considered as statistically significant.
Results
Effect of vitamin D deficiency on serum levels of immunoglobulin’s, C3, C4 complement proteins, C-reactive protein and rheumatoid factor: As shown in table 1, after twenty days of supplementation approximately three and four folds increase in mean of serum levels of vitamin D3 in male and female subjects respectively. Serum levels of IgM were significantly increased in male subjects after oral supplementation of vitamin D (28%) and further in females (31%) in which p values were <0.001 corresponding to baseline. That increase was accompanied with a slight increase in IgA, C4 and C3 complement proteins and exceptional significant increase in C3 in female subjects (p value was <0.05) corresponding to baseline. However, serum levels of IgG significantly decreased in female subjects in which p value was 0.05<corresponding to baseline. In addition, the data presented in table 1 also shows that there is no a clear change in both rheumatoid factor and CRP serum levels.
| Parameters (Mean ± SEM) | Male N (59) | Female N (42) | ||
|---|---|---|---|---|
| Baseline | Supplement | Baseline | Supplement | |
| Vitamin D level (ng/ml) | 12.73 ± 0.74 | 40.06 ± 2.52 | 10.85 ± 0.94 | 40.25 ± 0.96 |
| IgM (mg/dl) | 92.48 ± 8.2 | 128.32 ± 9.8** | 142.42 ± 14.3 | 206 ± 18.9** |
| IgG (mg/dl) | 1613 ± 84.7 | 1519 ± 109.6 | 2184 ± 99.2 | 1875 ± 92* |
| IgA (mg/dl) (Mean ± SEM) | 320 ± 33.9 | 427 ± 43.7 | 460 ± 37 | 466 ± 27.6 |
| C3 (mg/dl) | 171 ± 8.24 | 181 ± 10.6 | 205 ± 13.1 | 239 ± 7.8* |
| C4 (mg/dl) | 41.6 ± 3.75 | 47.6 ± 4.3 | 47.28 ± 3.25 | 57.5 ± 4.5 |
| RF (IU/ml) | 18.07 ± 0.3 | 17.1 ± 0.39 | 17 ± 0.46 | 16.9 ± 0.41 |
| CRP (mg/dl) | 0.55 ± 0.1 | 0.61 ± 0.01 | 0.92 ± 0.14 | 0.97 ± 0.2 |
Table 1. Serum levels of Vitamin D3, IgM, IgG, IgA, C3, C4, RF and CRP in Vitamin D deficient individuals (baseline) and after twenty days of (4000 IU) vitamin D3 oral administration for both Male and Female groups. Data are presented in mean ± SEM. ** indicates p values 0.001<corresponding to baseline and *indicates p values 0.05< corresponding to baseline.
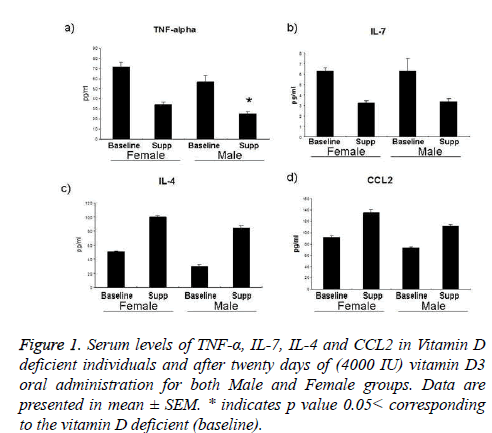
Differences in serum levels of TNF-α, IL-7, IL-4 and CCL2 between baseline (vitamin D deficient) and after twenty days of oral supplementation: Serum investigation data for all participants at baseline and after twenty days of oral intake of vitamin D3 shows a striking decrease in TNF-α levels in both female and male groups for about 47% and 44% respectively, and that was statistically significant in male participants in which p value was <0.05. Similar outcomes in interleukin-7 which was decreased for about 51% and 54% as indicated in figure 1b. Inversely, Interleukin-4 was increased for about 100% in female subjects and 175% in males and smaller changes in CCL2 results 47% in females and 52% increase in male participants.
Figure 1 : Serum levels of TNF-α, IL-7, IL-4 and CCL2 in Vitamin D deficient individuals and after twenty days of (4000 IU) vitamin D3 oral administration for both Male and Female groups. Data are presented in mean ± SEM. * indicates p value 0.05< corresponding to the vitamin D deficient (baseline).
Discussion
Nowadays, vitamin D deficiency became a phenomenon among population in Taif. The logic questions raised are to what extent lifestyle change can either improve or worsen the health status of individuals. In fact, vitamin D deficiency underlies several medical problems and thus this study became a very important step to ameliorate the public health in Saudi Arabia, Taif and over the world.
Current study clearly demonstrated that 4000 IU/day oral administration of vitamin D3 increases serum levels of vitamin from about 11 ng/ml to 40 mg/ml in male participants and similar ratio with female participants within twenty days which indicates the dosages strategy for vitamin D deficient adults. Table 1 shows different changes in serum levels of some humoral immunity components as a result of vitamin D3 elevation.
As shown in table 1 oral vitamin D3 supplementation reduced serum IgG levels for about 15% and 21.8 % in both male and female subjects respectively, and that was significant in females. In addition, serum IgM levels were significantly increased for about 38.7% and 44.6% in male and female subjects respectively. Serum levels of IgA, C3 and C4 were also increased after twenty days of oral supplementation in both sex groups. Consistent data was published in 1997 by Falkenbach and Seldmeyer where they found that exposure to ultra violet rays reduced serum IgG levels whereas increased serum levels of IgM, IgA and complement proteins [22]. Another study was performed on some chronic lung inflammation cases concluded that serum vitamin D3 levels are inversely associated with serum IgG levels [23].
Interleukin-7 is a lymphopoietic cytokines that required for peripheral lymphocyte homeostasis [24-26]. It is a potent regulator for T cell expansion and chronic inflammation and also plays a vital role in some autoimmune reactions [27-29]. In different context, IL-7 plays an inhibitory role in osteoclastogenesis [30]. Promising results are shown in figure 1b that oral vitamin D3 supplementation reduced serum IL-7 levels for about 50% in both male and female participants. These data might mean that vitamin D enhances immune response via its lymphopoietic role and might play other positive physiological roles in inflammation, osteoclastogenesis and as autoimmune preventer via IL-7 reduction. Similarly, vitamin D3 supplementation reduced TNF-α, a proinflammatory cytokine that is a lymphopoiesis stimulator as well [31-33]. Figure 1a clearly shows that serum TNF-α levels were reduced for about 47% in female subjects and significantly 44% in males after twenty days of vitamin D3 oral supplementation. Anti-inflammatory effect of vitamin D might be supported by data shown in figures 1a and 1d which tells that vitamin D supplementation increased serum interleukin-4 and CCL2 levels to about two folds in both male and female subjects [34-38].
Many studies have been elucidated the physiological importance of vitamin D3. One of such studies has mentioned that vitamin D insufficiency was major finding in patients with Grave’s disease [8]. Similarly, vitamin D deficiency is associated with rheumatoid arthritis, ankylosing spondylitis and systemic lupus erythematosus [7,10]. Moreover, Timms PM and his colleagues found that vitamin D supplementation reduced CRP and metalloproteinase-9 in healthy adults [39]. However, the current study does not show any significant changes in serum levels of both rheumatoid factor and Creactive protein after twenty days of oral supplementation and that might be due to either the very restricted criteria in participant selection or the positive effect of vitamin D via reducing both IL-7 and TNF-α levels and increasing the antiinflammatory cytokines IL-4 and CCL2.
Conclusion
We conclude that twenty days of 4000 IU of oral vitamin D3 supplementation can change vitamin D status from deficient to normal levels in individuals that assumed to be healthy. This elevation in turn, is accompanied with improvement in both innate and adaptive immunity and can prevent the autoimmune reaction via IL-7 reduction. In addition, vitamin D3 might serve as anti-inflammatory agent via reducing serum TNF-α and increasing serum levels of both IL-4 and CCL2. However, there is no change in serum levels of both rheumatoid factor and CRP that might be a consequence of serum cytokines change.
Acknowledgements
The authors would like to acknowledge and thank the Deans of Scientific Research Affairs in Taif University, Saudi Arabia for financial support of this study (project number 4092-36-1).
References
- Kulie T,Groff A,Redmer J,Hounshell J,Schrager S. Vitamin D: an evidence-based review. J Am Board Fam Med 2009; 22: 698-706.
- DeLuca HF. Vitamin D as a prohormone. BiochemPharmacol 1977; 26: 563-566.
- DeLuca HF. Overview of general physiologic features and functions of vitamin D. The American Journal of Clinical Nutrition, 2004; 80: 1689S-1696S.
- Holick MF,Binkley NC,Bischoff-Ferrari HA,Gordon CM,Hanley DA,Heaney RP,Murad MH,Weaver CM. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: an Endocrine Society Clinical Practice Guideline. Journal of Clinical Endocrinology & Metabolism 2011; 96: 1911-1930.
- Hayes CE, Cantorna MT, DeLuca HF. Vitamin D and Multiple Sclerosis. Experimental Biology and Medicine, 1997; 216: 21-27.
- Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H. The Role of Vitamin D in Cancer Prevention. American Journal of Public Health, 2006. 96(2): p. 252-261.
- Cantorna MT. Vitamin D and autoimmunity: is vitamin D status an environmental factor affecting autoimmune disease prevalence? Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine 2000. 223: 230-233.
- Yamashita H,Noguchi S,Takatsu K,Koike E,Murakami T,Watanabe S,Uchino S,Yamashita H,Kawamoto H. High prevalence of vitamin D deficiency in Japanese female patients with Graves' disease. Endocrine journal 2001; 48: 63-69.
- Falkenbach A, Tripathi R,Sedlmeyer A,Staudinger M,Herold M. Serum 25-hydroxyvitamin D and parathyroid hormone in patients with ankylosing spondylitis before and after a three-week rehabilitation treatment at high altitude during winter and spring. Wiener klinischeWochenschrift 2001; 113: 328-332.
- Huisman AM, White KP,Algra A,Harth M,Vieth R,Jacobs JW,Bijlsma JW,Bell DA. Vitamin D levels in women with systemic lupus erythematosus and fibromyalgia. The Journal of rheumatology 2001; 28: 2535-2539.
- Zhang Y, Leung DY,Richers BN,Liu Y,Remigio LK,Riches DW,Goleva E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. Journal of immunology 2012. 188: 2127-2135.
- Gruber HE, Hoelscher G,Ingram JA,Chow Y,Loeffler B,Hanley EN. 1,25(OH)2-vitamin D3 inhibits proliferation and decreases production of monocyte chemoattractant protein-1, thrombopoietin, VEGF, and angiogenin by human annulus cells in vitro. Spine 2008; 33: 755-65.
- Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. The American journal of clinical nutrition, 1999; 69: 842-856.
- Sinclair C. Risks and benefits of sun exposure: implications for public health practice based on the Australian experience. Progress in biophysics and molecular biology, 2006; 92: 173-178.
- Moan J, Porojnicu AC,Dahlback A,Setlow RB. Addressing the health benefits and risks, involving vitamin D or skin cancer, of increased sun exposure. Proceedings of the National Academy of Sciences of the United States of America, 2008; 105: 668-673.
- Sivamani RK, Crane LA, Dellavalle RP. The benefits and risks of ultraviolet tanning and its alternatives: the role of prudent sun exposure. Dermatologic clinics 2009; 27: 149-154.
- Nuhn P. Vitamin D - a Prohormone. Vitamin D and hyperparathyroidism. Pharm UnsererZeit 2009; 38: 136-139.
- Pittas AG, Harris SS,Stark PC,Dawson-Hughes B. The Effects of Calcium and Vitamin D Supplementation on Blood Glucose and Markers of Inflammation in Nondiabetic Adults. Diabetes Care 2007; 30: 980-986.
- Pettifor JM,Bikle DD,Cavaleros M,Zachen D,Kamdar MC,Ross FP. Serum levels of free 1,25-dihydroxyvitamin D in vitamin D toxicity. Annals of internal medicine 1995; 122: 511-513.
- Thomas MK, Demay MB. Vitamin D deficiency and disorders of vitamin D metabolism. Endocrinology and metabolism clinics of North America 2000; 29: 611-627.
- Sharma OP. VItamin d, calcium, and sarcoidosis. Chest 1996; 109: 535-539.
- Falkenbach A, Sedlmeyer A. Travel to sunny countries is associated with changes in immunological parameters. Photodermatologyphotoimmunology&photomedicine 1997; 13: 139-142.
- Pincikova T, Nilsson K,Moen IE,Karpati F,Fluge G,Hollsing A,Knudsen PK,Lindblad A,Mared L,Pressler T,Hjelte L.Scandinavian Cystic Fibrosis Study Consortium Inverse relation between vitamin D and serum total immunoglobulin G in the Scandinavian Cystic Fibrosis Nutritional Study. Eur J ClinNutr 2011; 65: 102-109.
- Namen AE,Lupton S,Hjerrild K,Wignall J,Mochizuki DY,schmierer A,mosley B,March CJ,urdal D,gillis S,cosman D,Goodwin RG. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature, 1988; 333: 571-573.
- Monroe JG, Allman D. Commentary: Keeping track of pro-B cells: a new model for the effects of IL-7 during B cell development. European Journal of Immunology 2004. 34: 2642-2646.
- Al-Shami A, Spolski R,Kelly J, Fry T,Pamela L, Akhilesh SP, Crystal LM,Warren JL. A Role for Thymic Stromal Lymphopoietin in CD4+ T Cell Development. The Journal of Experimental Medicine, 2004; 200: 159-168.
- Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nature reviews Immunology 2006; 6: 205-217.
- Bikker A, Aike AK, Kim MG van der Wurff-Jacobs ,Rogier PP, Marije K, Frank R, Wilco de J, Floris PJG Lafeber, Joël AG van Roon. Interleukin-7 and Toll-like receptor 7 induce synergistic B cell and T cell activation. PloS one 2014; 9: e94756.
- Ramanathan S, Gagnon J, Ilangumaran S. Antigen-nonspecific activation of CD8+ T lymphocytes by cytokines: relevance to immunity, autoimmunity, and cancer. ArchivumImmunologiae et TherapiaeExperimentalis 2008; 56: 311-323.
- Lee SK, Kalinowski JF,Jastrzebski SL,Puddington L,Lorenzo JA. Interleukin-7 Is a Direct Inhibitor of in Vitro Osteoclastogenesis. Endocrinology 2003; 144: 3524-3531.
- Robak T, Gladalska A, Stepien H. The tumour necrosis factor family of receptors/ligands in the serum of patients with rheumatoid arthritis. European cytokine network 1998; 9: 145-54.
- Ridker PM, Hennekens CH,Buring JE,Rifai N. C-Reactive Protein and Other Markers of Inflammation in the Prediction of Cardiovascular Disease in Women. New England Journal of Medicine 2000; 342: 836-843.
- Ryan DH,Nuccie BL,Ritterman I,LiesveldJL, Abboud CN. Cytokine regulation of early human lymphopoiesis. Journal of immunology 1994; 152: 5250-5258.
- Marie C, Pitton C,Fitting C,Cavaillon JM. Mediators of inflammation. 1996; 5: 334-340.
- Weiss L, Haeffner-Cavaillon N,Laude M,Cavaillon JM,Kazatchkine MD. Human T cells and interleukin 4 inhibit the release of interleukin 1 induced by lipopolysaccharide in serumfree cultures of autologous monocytes. European Journal of Immunology 1989. 19: 1347-1350
- Flaishon L, Hart G,Zelman E,Moussion C,Grabovsky V,Lapidot Tal G,Feigelson S,Margalit R,Harmelin A,Avin-Wittenberg T,Shoseyov D,Alon R,Girard JP,Shachar I. Anti-inflammatory effects of an inflammatory chemokine: CCL2 inhibits lymphocyte homing by modulation of CCL21-triggered integrin-mediated adhesions. Blood 2008. 112: 5016-5025.
- Wood S, VijayakumarJayaraman, Huelsmann EJ, Bonish B, Burgad D, Sivaramakrishnan G, Shanshan Qin, DiPietroLA, Zloza A, Zhang C, Shafikhani SH. PloS one 2014; 9: e91574.
- Ansari AW Hans Heiken,Meyer-Olson D, Schmidt RE. CCL2: a potential prognostic marker and target of anti-inflammatory strategy in HIV/AIDS pathogenesis. European Journal of Immunology, 2011. 41: 3412-3418.
- Timms PM, Mannan N,Hitman GA,Noonan K,Mills PG,Syndercombe-Court D,Aganna E,Price CP,Boucher BJ. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM : monthly journal of the Association of Physicians 2002; 95: 787-796.