Research Article - Current Pediatric Research (2021) Volume 25, Issue 9
Value of urinary Neutrophil Gelatinase-Associated Lipocalin (NGAL) in predicting acute kidney injury in neonates with perinatal asphyxia.
Walaa H Ali1*, Hanaa H Ahmed2, Hasanin M Hasanin3, lman H Kamel1, Wafaa O Ahmed4
1 Department of Child Health, National Research Centre, Dokki, Giza, Egypt
2 Department of Hormones, National Research Centre, Dokki, Giza, Egypt
3 Department of Pediatric, National Research Centre, Dokki, Giza, Egypt
4 Department of Pediatric, Ain Shams University, Abbasia, Egypt
- Corresponding Author:
- Walaa H Ali
Department of Child Health
National Research Centre
Dokki
Giza, Egypt
Tele: +201126537401
E-mail: walaa.hani79@gmail.com
Accepted date: 21st September, 2021
Abstract
Background: Acute Kidney Injury (AKI) affects 30%-55% of asphyxiated neonates, with a 60%-66% mortality rate. Because novel biomarkers measured at the time of Intensive Care Unit (ICU) admission have been shown to predict short and long-term outcomes, the purpose of this study was to assess the role of urinary Neutrophil Gelatinase-Associated Lipocalin (NGAL) as an early biomarker for detecting Acute Kidney Injury (AKI) in neonates with perinatal asphyxia. Methods: This study included 91 full-term neonates (45 of whom were asphyxiated and 46 of whom were not). The asphyxiated group was subdivided further into AKI and non-AKI groups. UNGAL was measured 6 hours after birth, while CRP, creatinine, potassium, and blood urea nitrogen levels were measured 48 hours later. The biomarkers' diagnostic value was determined using Receiver Operating Characteristic (ROC) curves. Results: In terms of age and gender, there was no significant difference between the two groups (asphyxiated and non-asphyxiated). In contrast, there was a significant difference between the two groups in terms of the need for resuscitation, the need for oxygen support, the need for ventilation, the need for total parenteral nutrition, and the mode of delivery, with a P value of (<0.001, < 0.001, <0.001, <0.001 and 0.018 respectively). The results of laboratory tests (uNGAL, potassium, blood urea nitrogen, and creatinine) were significantly higher in the asphyxiated group than in the non-asphyxiated group, with a P value of (<0.001, 0.03, <0.001 and <0.001 respectively). The non-asphyxiated group had significantly higher Apgar scores at one and five minutes, urine output, albumin, and base excess than the asphyxiated group, with a P value of (<0.001, <0.001, <0.001 and 0.012 respectively). In the asphyxiated group with AKI, uNGAL showed a significant positive correlation with serum creatinine (P<0.001) and a significant negative correlation with (pH, base excess, and Apgar score at (1min-5 min) with P value (<0.001, 0.021, and <0.001 respectively). Conclusion: As a result, early detection of uNGAL as a novel and non-invasive biomarker can predict the occurrence of AKI in neonates with perinatal asphyxia, allowing for early intervention and the avoidance of complications.
Keywords
Neonates, Asphyxia, Acute kidney injury, Urinary biomarker, Neutrophil gelatinase-associated lipocalin.
Introduction
Asphyxia is a major cause of Neonatal Intensive Care Unit (NICU) admission and the second leading cause of morbidity and mortality in term and preterm neonates. In poor countries, the prevalence of asphyxia ranges from 5 to 10 per 1000 live births [1]. The hypoxic ischemic phenomenon does not always occur after birth, accounting for only 10% of all cases. The majority of cases occur before birth (20%) and during delivery (33%), respectively [2].
Acute Kidney Injury (AKI) affects 30%-55% of asphyxiated neonates, with a 60%-66% mortality rate [3]. AKI has largely replaced Acute Renal Failure (ARF) because it better defines renal dysfunction as a continuum rather than a discrete finding of failed kidney function. AKI is defined as a sudden loss of kidney function that results in a decrease in Glomerular Filtration Rate (GFR), retention of urea and other nitrogenous waste products, and dysregulation of extracellular volume and electrolytes [4].
Diagnosis of AKI in neonates is difficult because most clinical and biochemical parameters are independent at this age [1]. Serial measurements of kidney function biomarkers such as serum creatinine are currently used to make the diagnosis in approximately 30% of critically ill neonates and children who suffer from AKI and its consequences [5,6]. Serum creatinine is frequently a delayed and imprecise test because it reflects GFR in individuals who are in a steady state with stable kidney function and does not accurately reflect GFR in a patient whose renal function is changing.
A neonate in the early stages of severe AKI with a markedly reduced GFR, for example, may have a relatively normal or slightly elevated creatinine because there hasn't been enough time for creatinine accumulation [7]. Novel AKI biomarkers have the potential to not only diagnose AKI earlier than changes in serum creatinine, but also to predict the development of AKI, distinguish the aetiology of the AKI, and predict the likelihood of adverse post-AKI short and long-term outcomes [8].
Neutrophophil Gelatinase-Associated Lipocalin (NGAL), Kidney Injury Molecule 1 (KIM-1), Interleukin 18 (IL-18), Liver-type Fatty Acid-Binding Protein (L-FABP), neutrophil Elastase-2 (Ela-2) and Cystatin C (Cys-C) are some of these biomarkers [9,10]. NGAL is a lipocalin family protein that is widely expressed and serves as a bacteriostatic agent in the innate immune response. Following renal injury, NGAL production is dramatically increased [11]. The purpose of this study was to determine the benefit of uNGAL in the early detection of AKI in neonates exposed to perinatal asphyxia. This study was expanded to assess the sensitivity and specificity of uNGAL cutoff values in the diagnosis of AKI in these neonates.
Subjects and Methods
Patients and study design
This comparative cross-sectional study included 91 full-term neonates admitted to the NICU of Ain Shams university’s pediatric hospital over a four-month period (March 2021-July 2021). According to the inclusion criteria, the neonates were divided into two groups: 45 neonates with a history of perinatal asphyxia (asphyxiated group) and 46 neonates (age and sex matched) admitted to the Neonatal Intensive Care Unit (NICU) for reasons other than asphyxia (non-asphyxiated group). The asphyxiated cases included in this study were chosen in accordance with the Chinese medical association perinatal medicine branch. Three or more of the following symptoms indicate perinatal asphyxia: (a) The pH<7.0 in umbilical artery, (b) Apgar score <6 at 5 minute, (c) meconium stained liquor and (d) changes in fetal heart rate [1].
The neonates in the non-asphyxiated group were incubated for reasons other than hypoxia. The group of asphyxiated cases was further subdivided 48 hours after NICU admission into asphyxiated neonates with AKI (n=18) and asphyxiated neonates without AKI (n=27). AKI in neonates was defined as having oliguria (<0.5 mL/kg/hr) as well as elevated serum creatinine (>1.5 mg/dL) for more than 48 hours [12]. The study excluded neonates with a history of congenital renal anomalies or congenital infection demonstrated by antenatal or postnatal care, neonates with a history of maternal kidney disease, neonates who had received nephrotoxic drugs, and preterms.
Methods: All neonates who took part in the study had a complete history taken from their parents, including antenatal, maternal, obstetric, and postnatal history. A thorough clinical examination of the neonates was performed in order to determine system failure, with special emphasis on neurological and urological estimation. All neonates had their Urine Output (UOP) measured every 24 hours using a plastic collection bag. Each neonate had a venous blood sample of 3 ml drawn using sterile equipment. An aliquot (1 ml) was collected in tubes containing EDTA as an anticoagulant, while the remaining 2 ml was collected in two tubes without anticoagulant to separate sera using cooling centrifugation at 1800 xg for 10 min at 4°C and the aliquots of serum samples were stored at -20°C till the time of analysis.
Laboratory investigations for the neonates were carried out in the form of Complete Blood Count (CBC) including hemoglobin, RBC, WBC, platelets counts by using an automated analyzer (Cel-dyn.3500; Abbott Diagnostics, Abbott park; IL). Semi quantitative C Reactive Protein (CRP) was analyzed by latex agglutination (omega diagnostic, Ltd, Alva, UK) following the manufacturer's instruction. Serum creatinine was determined by bio systems reagent kit provided by bio systems SA (Barcelonan, Spain) by modified Jaff reaction. Serum blood urea nitrogen was estimated by the enzymatic colorimetric test, using a diamond kit (diamond diagnostic, Holliston, USA) following manufacturer's manual. Serum albumin was quantified using the bromocresol green method (Ortho-Clinical Diagnostics Inc., Rochester, NY, USA) following the manufacturer's recommendation. Sodium and potassium were analyzed by colorimetric method (Lab Care Diagnostics, Pvt, Ltd, India) as recommended by manufacturer's protocol. The laboratory evaluations were determined after 48 hours of NICU admission. Urine samples (10 ml) were collected to measure NGAL by human neutrophil gelatinase-associated ELISA kit (Sino Gene Clon Biotech Co., Ltd) as a marker of AKI following the manufacturer's instruction. The uNGAL level was estimated after 6 hours of NICU admission. Radiological investigations such as chest X-ray, echocardiography and abdominal ultrasound were done for diagnosing and excluding associated anomalies.
Ethical approval: This study was approved by medical research ethical committee of the national research centre, Egypt. A written informed consent for all participants was collected from their parents after explaining the purpose and methodology of the study.
Statistical analysis
The collected data was revised, coded, tabulated and introduced to a PC using Statistical Package for Social Science (SPSS 20 for windows). Data cleaning and checking for quality of data and data entry was performed. Data was presented and suitable analysis was done according to the type of data obtained for each parameter.
Descriptive statistics include mean, Standard Deviation (SD), and frequency and percentage for parametric numerical data, as well as frequency and percentage for qualitative data. Analytic statistics, on the other hand, include the student t-test, which was used to determine the statistical significance of the difference between two study group means, and the chi-square test, which was used to investigate the relationship between two qualitative variables. When the expected count is less than 5 in more than 20% of cells, Fisher's exact test was used to investigate the relationship between two qualitative variables. Pearson correlation was used to determine the strength of relationship between two quantitative variables. The ROC curve and area under the curve were calculated to test the value of NGAL and creatinine in the diagnosis of AKI in asphyxiated neonates. Accordingly coordinates of the curve were done to determine the cut off values with sensitivity and specificity.
Results
In total, 91 neonates participated in the current study. They were divided into two categories: The first group is made up of 45 asphyxiated neonates (asphyxiated group), and the second group is made up of 46 non-asphyxiated neonates (non-asphyxiated group). Table 1 depicts the characteristics of all neonates in the study as well as their mothers (1). In terms of sex and gestational age, there was no statistically significant difference between asphyxiated and non-asphyxiated neonates. The cases had 60.9% males and 39.1% females, while the controls had 64.4% males and 35.6% females (p=0.06).
The mean gestational age among asphyxiated neonates was 39.09 ± 1.2, but among non-asphyxiated neonates was 39.3 ± 1.3 (P=0.737). The mean birth weight of asphyxiated group was 2476.8 ± 948.1 g and the mean birth weight of non-asphyxiated group was 2477.7 ± 934.5 g, with insignificant difference between the two groups (p=0.99).
Approximately 62.2% of asphyxiated neonates had a history of multiple pregnancies, whereas 76% of non-asphyxiated neonates had a single pregnancy. Approximately 82.6% of neonates with asphyxia had a history of antenatal maternal disease. There was a significant difference between asphyxiated and non-asphyxiated neonates in terms of need for resuscitation at delivery, oxygen support, ventilation, Total Parenteral Nutrition (TPN), and mode of delivery (p<0.001, <0.001, <0.001, <0.001 and<0.018, respectively).
Apgar scores (1 and 5 minutes) and Urine Output (UOP) revealed a statistically significant difference (p<0.001) between asphyxiated and non-asphyxiated groups, with higher mean values in the non-asphyxiated group.
| Variables | Asphyxiated neonates N=45 n (%) | Non-asphyxiated neonates N=46 n (%) | p value |
|---|---|---|---|
| Gestational age (weeks) | 39.09 ± 1.2 | 39.3 ± 1.3 | 0.737 |
| Sex | |||
| Male | 28 (62.2%) | 29 (63%) | 0.06 |
| Female | 17 (37.8%) | 16 (27%) | |
| Weight (g) (mean ± SD) | 2476.78 ± 948.13 | 2477.72 ± 934.48 | 0.99 |
| Delivery mode | |||
| CS | 28 (62.2%) | 38 (82.6%) | 0.018* |
| Vaginal | 17 (37.8%) | 8 (17.4%) | |
| Single or multiple pregnancy | |||
| Single | 17 (37.8%) | 35 (76%) | <0.001* |
| Multiple | 28 (62.2%) | 11 (24%) | |
| Antenatal maternal disease | |||
| Yes | 38 (82.6%) | 18 (40%) | <0.001* |
| No | 7 (27.4%) | 28 (60%) | |
| Need to resuscitation at delivery | |||
| Yes | 35 (77.7%) | 16 (34.8%) | <0.001* |
| No | 10 (32.3%) | 30 (65.2%) | |
| Need to oxygen support | |||
| Yes | 41 (91.1%) | 12 (26.1%) | <0.001* |
| No | 4 (8.9%) | 34 (73.9%) | |
| Need to ventilation | |||
| Yes | 29 (64.4%) | 9 (19.6%) | <0.001* |
| No | 16 (35.6%) | 37 (81.4%) | |
| Need for TPN | |||
| Yes | 28 (62.2%) | 11 (24%) | <0.001* |
| No | 17 (37.8%) | 35 (76%) | |
| Apgar at 1 minute (mean ± SD) | 2.84 ± 1.74 | 6.7 ± 0.6 | <0.001* |
| Apgar at 5 minute (mean ± SD) | 4.87 ± 1.05 | 7.5 ± 0.5 | <0.001* |
| Urine output (cc/kg/hour) (mean ± SD) | 1.94 ± 0.41 | 3.08 ± 0.71 | <0.001* |
Table 1. Comparison between asphyxiated group and non-asphyxiated group (non-asphyxiated neonates) regarding clinical data. *: p<0.05 significant.
The mean values of uNGAL, potassium, creatinine, and blood urea nitrogen in asphyxiated neonates were significantly higher than in non-asphyxiated neonates (p<0.001, <0.03, <0.001, and <0.001, respectively). The asphyxiated neonates had a non-significant (p=0.06) increase in serum CRP compared to their non-asphyxiated counterparts. On the contrary, the mean values of albumin, umbilical artery pH, and base excess were significantly higher in the non-asphyxiated group than in the asphyxiated group (p<0.001, <0.001, and <0.012, respectively). The difference in serum sodium levels between the asphyxiated and non-asphyxiated groups was insignificant (p=0.07) (Table 2).
| Variables | Asphyxiated neonates N=45 mean ± SD | Non-asphyxiated neonates N=46 mean ± SD | p value |
|---|---|---|---|
| Hemoglobin (g/dl) | 13.78 ± 5.4 | 13.56 ± 3.51 | 0.8 |
| Total leukocytic count (× 109/L) | 12.69 ± 3.01 | 13.1 ± 3.37 | 0.5 |
| Platelets (× 109/L) | 198.82 ± 134.11 | 248.87 ± 183.26 | 0.09 |
| Albumin (g/dl) | 2.6 ± 1.07 | 3.5 ± 0.68 | <0.001* |
| CRP (mg/dl) | 34.2 ± 32.11 | 26.29 ± 18.37 | 0.06 |
| NGAL (ng/ml) | 7.22 ± 1.16 | 2.32 ± 0.32 | <0.001* |
| Sodium (meq/L) | 139.43 ± 6.36 | 142.43 ± 8.16 | 0.07 |
| Potassium (meq/L) | 4.60 ± 1.35 | 4.06 ± 1.08 | 0.03* |
| Creatinine (mg/dl) | 1.7 ± 1.29 | 0.36 ± 0.14 | <0.001* |
| Blood urea nitrogen (mg/dl) | 56.08 ± 25.17 | 28.36 ± 5.74 | <0.001* |
| pH of umbilical artery | 7.09 ± 0.28 | 7.4 ± 0.04 | <0.001* |
| Base excess | -6.37 ± 9.47 | -2.54 ± 3.6 | 0.012* |
Table 2. Comparison between the asphyxiated and non-asphyxiated neonates regarding the laboratory investigations. *: p<0.05 significant.
Table 3 shows that there was a significant difference in urine output and delivery mode between asphyxiated cases with AKI and asphyxiated cases without AKI (P=0.04, <0.001 respectively).
| Variables | AKI N=18 N (%) | Non AKI N=27 N (%) | p value |
|---|---|---|---|
| Sex | |||
| Male | 10 (55.6%) | 19 (70.4%) | 0.56 |
| Female | 8 (44.4%) | 8 (29.6%) | |
| Weight (g) (mean ± SD) | 2814.17 ± 1058.32 | 2251.85 ± 810.93 | 0.05 |
| Delivery mode | <0.001* | ||
| CS | 14 (77.8%) | 24 (88.9%) | |
| Vaginal | 4 (22.2%) | 3 (11.1%) | |
| Single or multiple pregnancy | 0.37 | ||
| Single | 6 (33.3%) | 11 (40.7%) | |
| Multiple | 12 (66.7%) | 16 (59.3%) | |
| Need to ventilation | 0.67 | ||
| Yes | 18 (100%) | 11 (40.7%) | |
| No | 16 (40.3%) | ||
| Need for TPN | 0.72 | ||
| Yes | 18 (100%) | 10 (37%) | |
| No | 17 (63%) | ||
| Apgar at 1 minute (mean ± SD) | 2.84 ± 1.74 | 6.7 ± 0.6 | 0.9 |
| Apgar at 5 minute (mean ± SD) | 4.87 ± 1.05 | 7.5 ± 0.5 | 0.3 |
| Urine Output (cc/kg/hour) (mean ± SD) | 1.6 ± 1.44 | 2.16 ± 1.37 | 0.04* |
Table 3. Comparison between the AKI asphyxiated neonates and non-AKI asphyxiated neonates regarding clinical data. *: p<0.05 significant.
Urinary NGAL, CRP, potassium, creatinine and blood urea nitrogen had significant higher mean values in AKI asphyxiated cases than non AKI asphyxiated cases (p=0.04, 0.04, <0.001, <0.001 and <0.001 respectively). Whereas serum albumin had significant higher level in non-AKI asphyxiated cases than AKI asphyxiated cases (Table 4).
| Variable | AKI N=18 mean ± SD | Non-AKI N=27 mean ± SD | p value |
|---|---|---|---|
| Hemoglobin (g/dl) | 13.7 ± 6.24 | 13.91 ± 4.18 | 0.9 |
| Total leukocytic count (× 109/L) | 13.93 ± 3.26 | 11.86±2.57 | 0.02 |
| Platelets (× 109/L) | 203.39 ± 166.55 | 195.78 ± 128.45 | 0.86 |
| Albumin (gm/dl) | 1.84 ± 0.48 | 3.87 ± 0.15 | <0.001** |
| CRP (mg/dl) | 40.33 ± 38.88 | 19.78 ± 20.78 | 0.04* |
| NGAL (ng/ml) | 7.27 ± 1.24 | 5.13 ± 1.05 | 0.04* |
| Sodium ( meq/L) | 138.88 ± 4.4 | 144.48 ± 9.33 | 0.02 |
| Potassium (meq/L) | 5.32 ± 1.26 | 3.51 ± 0.42 | <0.001* |
| Creatinine (mg/dl) | 1.66 ± 0.65 | 0.59 ± 0.19 | <0.001* |
| Blood urea nitrogen (mg/dl) | 75.07 ± 10.85 | 27.61 ± 5.39 | <0.001* |
| pH of umbilical artery | 7.09 ± 0.27 | 7.1 ± 0.29 | 0.9 |
Table 4. Comparison between the AKI asphyiated neonates and Non-AKI asphyxiated neonates regarding the laboratory investigations. *: p<0.05 significant.
Table 1 shows the correlations of NGAL with other clinical and laboratory data (Table 5). UNGAL was found to have a significant negative correlation with umbilical artery pH, base excess, and Apgar score at 1 and 5 minutes (p<0.001,0.021,<0.001, and <0.001, respectively). uNGAL, on the other hand, showed a significant positive correlation with creatinine (p<0.001).
| Parameters | NGAL | |
|---|---|---|
| Age | Pearson Correlation ( r ) | 0.072 |
| P value | 0.497 | |
| Weight | Pearson Correlation ( r ) | -0.033 |
| P value | 0.759 | |
| Total leukocytic count | Pearson Correlation ( r ) | -0.028 |
| P value | 0.791 | |
| Hb | Pearson Correlation ( r ) | 0.033 |
| P value | 0.759 | |
| Platelets | Pearson Correlation ( r ) | -0.185 |
| P value | 0.079 | |
| CRP | Pearson Correlation ( r ) | 0.200 |
| P value | 0.057 | |
| pH of umbilical artery | Pearson Correlation ( r ) | -0.592 |
| P value | <0.001* | |
| Base excess | Pearson Correlation ( r ) | -0.242 |
| P value | 0.021* | |
| Apgar at 1 minute | Pearson Correlation ( r ) | -0.803 |
| P value | <0.001* | |
| Apgar at 5 minute | Pearson Correlation ( r ) | -0.791 |
| P value | <0.001* | |
| Urine output | Pearson Correlation ( r ) | 0.21 |
| P value | 0.403 | |
| Blood urea nitrogen | Pearson Correlation ( r ) | -0.091 |
| P value | 0.719 | |
| Creatinine | Pearson Correlation ( r ) | 0.458 |
| P value | <0.001* | |
Table 5. Spearman correlations of NGAL with other laboratory and clinical data in asphyxiated neonates with AKI. *: Significant correlation at p<0.05.
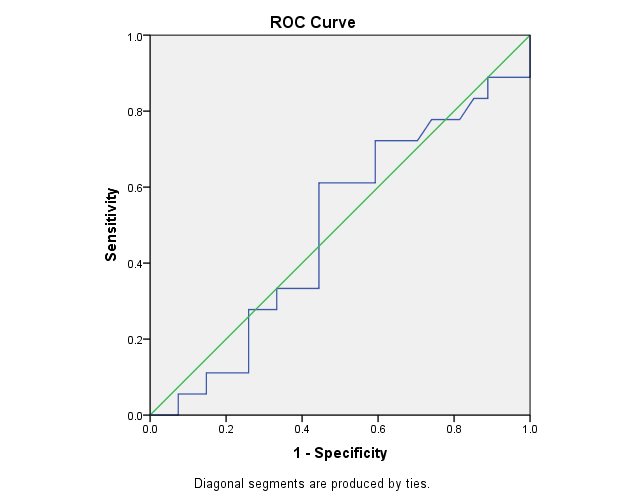
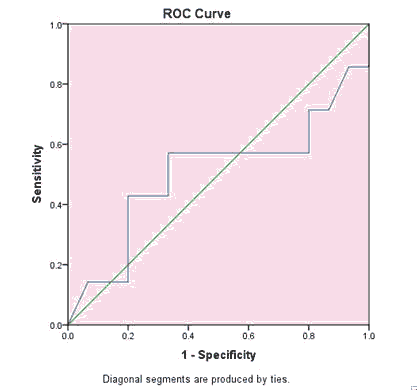
Figures 1 and 2 showed that NGAL sensitivity was 74.1% and specificity was 72.2%, at cutoff value 6.3 and AUC 0.49. Creatinine sensitivity was 71% and specificity was 80%, at cutoff value 0.3 and AUC 0.505 for detection of AKI in asphyxiated neonates (Table 6).
| Area | Std. Errora | Asymptotic Sig.b | Asymptotic 95% confidence interval | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| 0.49 | 0.089 | 0.908 | 0.316 | 0.664 |
Table 6. Test result variable(s): NGAL. a: Under the nonparametric assumption; b: Null hypothesis: true area=0.5.
The test result variable(s): NGAL had at least one tie between the positive actual state group and the negative actual state group. Statistics may be biased (Tables 7-9).
| AUC | Cut off value | Specificity | Sensitivity |
|---|---|---|---|
| 0.49 | 6.3 | 72.20% | 74.10% |
Table 7. ROC curve analysis for using creatinine in detection of AKI in neonates with asphyxia.
| Area | Std. Errora | Asymptotic Sig.b | Asymptotic 95% Confidence Interval | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| 0.505 | 0.151 | 0.972 | 0.208 | 0.801 |
Table 8. Test result variable(s): Creatinine. a: Under the nonparametric assumption; b: Null hypothesis: true area=0.5.
| AUC | Cut off value | Specificity | Sensitivity |
|---|---|---|---|
| 0.505 | 0.3 | 71% | 80% |
Table 9. The test result variable(s): Creatinine had at least one tie between the positive actual state group and the negative actual state group. Statistics may be biased.
Discussion
Asphyxia is a leading cause of NICU admission, and acute kidney injury affects 30%-55% of asphyxiated neonates, with a mortality rate of 60%-66% [3]. Novel biomarkers detected during Intensive Care Unit (ICU) admission have been shown to predict both short and long-term outcomes [13,14]. In this study, we looked at a novel biomarker; uNGAL in the urine in addition to routine laboratory tests to predict the development of AKI in neonates with perinatal asphyxia and to anticipate early intervention for these high-risk patients.
There was no significant difference in gestational age, sex, or birth weight between the asphyxiated and non-asphyxiated groups of neonates in our study. This is consistent with the findings of a study conducted by Zhang et al. [1]. However, Gopal et al. [15] study found that the majority of asphyxiated neonates were males. 62.2% of asphyxiated group mothers had a history of multiple pregnancies, and 82.6% had antenatal maternal disease. These findings are consistent with those of Liborio et al. who found that 27 of 46 neonates with AKI (58.7%) had multiparous mothers, with 31 (67.3%) of those mothers receiving regular antenatal care due to an antenatal disease [16].
Selewski et al. found a significant difference in instrumental vaginal delivery and urine output with p values of (<0.001 and 0.01 respectively) in 55 of 120 asphyxiated neonates with AKI in their study [17]. These findings are consistent with our findings, in which there was a significant difference between the asphyxiated group with AKI and the asphyxiated group without AKI in terms of mode of delivery and urine output, with p values of (<0.001 and 0.04 respectively). Furthermore, the need for resuscitation, oxygen support, ventilation, and TPN was significantly higher in asphyxiated neonates than in non-asphyxiated counterparts with p value of (<0.001 and 0.04 respectively)
Because of the pulmonary effects of hypoxia, such as pulmonary hypertension, pulmonary haemorrhage, and pulmonary edoema caused by heart failure or the inability of the lungs to produce surfactant, either non-invasive or invasive oxygen support is required to moderate both oxygen and carbon dioxide levels. Also, hypoxia damages the gut wall, making it impossible to introduce enteral feeding, so TPN is usually initiated [18].
Renal impairment, including increased blood urea nitrogen, creatinine, and decreased urine output, is a common finding in neonates with perinatal asphyxia [19]. The asphyxiated neonates had significantly higher levels of uNGAL (6 hours postnatal) as well as potassium, creatinine, and blood urea nitrogen (2 days postnatal) than the non-asphyxiated group, with p values of (<0.001, 0.03, <0.001, and <0.001 respectively). In addition, CRP levels (2 days postnatal) showed a non-significant increase in the asphyxiated group compared to the non-asphyxiated group (p=0.06).
This finding is consistent with that of Sarafidis et al. who discovered that serum and urinary levels of NGAL were significantly higher in asphyxiated neonates on days 1, 3 and 10 of life compared to controls regardless of serum creatinine elevation [20]. This reflects NGAL's role as an AKI diagnostic marker. Also, our findings are consistent with those of Askenazi et al. who found an increase in serum creatinine, blood urea nitrogen, and uNGAL in 110 asphyxiated neonates when compared to controls, with significant p values of 0.05, 0.01 and <0.001, respectively [21].
In perinatal asphyxia, disturbed acid-base balance (metabolic acidosis) is a common finding [22]. A pH of <7 indicates a 50% chance of an abnormal outcome, including neonatal sepsis, hypoalbuminemia, and neurological sequences. This is consistent with our findings, in which the non-asphyxiated group had significantly higher levels of albumin, umbilical artery pH, and base excess than the asphyxiated group, with p values of (<0.001, <0.001, and 0.012, respectively).
AKI is a common consequence in asphyxiated neonates compared to asphyxiated neonates without AKI with significantly higher levels of (uNGAL, CRP, potassium, creatinine, and blood urea nitrogen) with corresponding p values (0.04, 0.04, <0.001, <0.001, and <0.001 respectively). Tanigasalam et al. found AKI in 55 of 120 asphyxiated neonates (46 %) [23]. Furthermore, our findings are consistent with those of Amardiyanto et al. who found AKI in 18 of 32 asphyxiated neonates (56.25%) with significant high levels of serum urea and creatinine on the third day of life [24].
Serum creatinine is a marker of kidney function rather than kidney damage. After a 50% reduction in GFR, a 48-72 hrs delay in its rise was observed. As a result, urinary NGAL levels are a novel biomarker for predicting the occurrence of AKI [25]. In our study of asphyxiated neonates with AKI, uNGAL was found to be positively correlated with serum creatinine, with a significant p value of <0.001.
Perinatal asphyxia is associated with a poor prognosis, poor outcome, and a higher mortality rate, particularly in low birth weight neonates, as well as increased NICU admission duration, oxygen requirements, and ventilation. Alaro et al. found that uNGAL was negatively correlated with umbilical artery pH, base excess, and Apgar score, with significant P values of (<0.001, 0.021, and< 0.001, respectively) [26].
In our study, the serum creatinine cutoff point was 0.3 mg/dl, with 71% sensitivity and 80% specificity. El-Gasmy reported that in asphyxiated neonates with AKI, creatinine sensitivity was 41.5% and specificity was 52.7% at a cutoff point of 0.5 mg/dl [27]. In our study, we found that at a cutoff point of 6.3 ng/ml, the sensitivity and specificity of uNGAL for AKI prediction in asphyxiated neonates were 74.1% and 72.2% respectively. Similarly; uNGAL sensitivity and specificity were 84% and 88%, respectively, in a study conducted by Zhang et al. [1].
Conclusion
In the current study, 40% of asphyxiated neonates had AKI, and their uNGAL was significantly higher than in the asphyxiated neonates who did not have AKI. uNGAL also had a positive correlation with serum creatinine but a negative correlation with pH, base excess, and Apgar score. As a result, early detection of uNGAL as a novel, non-invasive biomarker aids in the prediction of AKI in neonates with perinatal asphyxia, allowing for early intervention and prevention of complications, as well as a reduction in neonatal mortality.
Acknowledgment
Authors are thankful to the National Research Centre and the Pediatric Hospital, Ain Shams University for their full support to carry out this study.
Conflict of interest
All authors declared that they have no conflict of interest.
References
- Zhang Y, Zhang B, Wang D, et al. Evaluation of novel biomarkers for early diagnosis of acute kidney injury in asphyxiated full-term newborns: A case control study. Med Prin Pract 2020; 29: 285-91.
- Glass HC, Ferriero DM. Treatment of hypoxic-ischemic encephalopathy in newborns. Curr Treat Options Neurol 2007; 9(6): 414-23.
- Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systemic review of maternal mortality and morbidity. Bull World Organ 2010; 88(1): 31-8.
- Devarajan P. Pediatric acute kidney injury: different from acute renal failure but how and why. Curr Pediatr Rep 2013; 1(1):34-40.
- El-Wakeel MA, El-Kassas GM, Fathy GA, et al. Diagnostic and prognostic values of high sensitive c-reactive protein, tumor necrosis factor and interleukin-1β in neonatal sepsis. Aust J Basic Appl Sci 2012; 6: 224-8.
- Ciccia E, Devarajan. Pediatric acute kidney injury, prevalence, impact and management challenges. Int J Nephrol Renovasc Dis 2017; 10: 77-84.
- Kaddourah A, Basu RK, Goldstein SL, et al. Oliguria and acute kidney injury in critically ill children: Implications for diagnosis and outcomes. Pediatr Crit Care Med 2019; 20(4): 332-9.
- Jason H, Chirag R. Biomarkers for diagnosis and prognosis of aki in children, one size does not fit all. Clin J Am Soc Nephrol 2017; 12(9): 1551-7.
- Rizvi M, Kashani KB. Biomarkers for early detection of acute kidney injury. J Appl Lab Med 2017; 2(3): 386-99.
- El Wakeel MA, Sabry RN, El-Kassas GM, et al. Pentraxin 3: A potential novel predictor for neonatal pulmonary hypertension. Open Access Maced J Med Sci. 2019; 7(15): 2424-2427.
- Dai X, Zeng Z, Fu C, et al. Diagnostic value of neutrophil gelatinase-associated lipocalin, cystatin C and soluble triggering receptor expressed on myeloid cells-1 in critically ill patients with sepsis associated acute kidney injury. Crit Care 2015; 19(1): 223
- Jetton JG, Askenazi DJ. Acute kidney injury in the neonates. Clin Perinatol J 2014; 41(3): 487-502.
- Srisawat N, Kulvichit W, Mahamitra N, et al. The epidemiology and characteristics of acute kidney injury in the Southeast Asia intensive care unit: A prospective multicentre study. Nephrol Dial Transplant. 2020; 35(10): 1729-38.
- El-Kassas GM, El Wakeel MA, Elabd MA, et al. Vitamin D status in neonatal pulmonary infections: relationship to inflammatory indicators. Open Access Maced J Med Sci 2019; 7(23): 3970-4.
- Gopal G. Acute Kidney Injury (AKI) in perinatal asphyxia. Indian J Pharm Biol Res J 2014; 2(2): 60-65.
- Libório AB, Castello Branco KMP, de Melo Bezerra CT. Acute kidney injury in neonates: From urine output to new biomarkers. Biomed Res Int 2014; 45: 221-3.
- Selewski DT, Jordan BK, Askenazi DJ, et al. Acute kidney injury in asphyxiated newborns treated with therapeutic hypothermia. J Pediatr 2013; 162(4):725-9.
- Vohra R, Singh V, Bansal, et al. Respiratory and gastrointestinal involvement in birth asphyxia. Acc J Pediatr Neonatol 2018; 6(4): 74-8.
- Nouri S, Mahdhaoui N, Beizig S, et al. Acute renal failure in full term neonates with perinatal asphyxia. Arch Pediatr 2008; 15(3): 229-35.
- Sarafidis K, Tsepkentzi E, Agakidou E, et al. Serum and urine acute kidney injury biomarkers in asphyxiated neonates. Pediatr Nephrol. 2012.27(9):1575-1582.
- Askenazi DJ, Ambalavanan NS, Goldstein SL. Acute kidney injury in critically ill newborns: What do we know? What do we need to learn?. Pediatric Nephrol. 2009; 24(2): 265-74.
- Rorbye C, Perslev A, Nickelsen C. Lactate versus pH levels in fetal scalp blood during labor-using the lactate scout system. J Matern Fetal Neonatal Med 2016; 29(8):1200-4.
- Tanigasalam V, Bhat BV, Adhisivam B, et al. Predicting severity of acute kidney injury in term neonates with perinatal asphyxia using urinary neutrophil gelatinase associated lipocalin. Indian J Pediatr 2016; 83(12-13): 1374-8.
- Amardiyanto R, Trihono PP, Rundjan, et al. Acute Kidney injury in Asphyxiated neonates. Paediatr Indones 2013; 53(4): 232-8
- Rakesh K, Bhat BV, Adhisivam B, et al. Effect of therapeutic hypothermia on myocardial dysfunction in term neonates with perinatal asphyxia-a randomized controlled trial. J Matern Fetal Neonatal Med 2018; 31(18): 2418-23.
- Alaro D, Bashir A, Mouske R, et al. Prevalence and outcomes of acute kidney injury in term neonates with perinatal asphyxia. Afr Health Sci 2014; 14(3): 682-8.
- El-Gasmy MA. Early predictors of Acute Kidney Injury (AKI) in a sample of Egyptian full-term neonates. Med Clin Rev 2017; 3(12): 1-6.