Research Article - Biomedical Research (2017) Volume 28, Issue 17
Value of the neutrophil CD64 index for diagnosing secondary infection in severe acute pancreatitis patients
Hui Fan, Yufeng Liu, Weisong Xu and Xiaohui Ni*
Department of Gastroenterology, the Second People’s Hospital of Nantong City, Jiangsu, PR China
- *Corresponding Author:
- Xiaohui Ni
Department of Gastroenterology
The Second People’s Hospital of Nantong City, PR China
Accepted on August 22, 2017
Abstract
Objective: This study aimed to investigate the value of the neutrophil CD64 index for diagnosing secondary infection in Severe Acute Pancreatitis (SAP) patients.
Methods: A total of 43 patients with SAP were enrolled between January 2011 and December 2015. Patient Acute Physiology and Chronic Health Evaluation II and Rason scores were calculated and blood samples were collected to determine the neutrophil CD64 index using flow cytometry. White blood cell counts and C-reactive protein levels were also determined for all patients. Blood culture (the gold standard for the diagnosis of infection subsequent to SAP) and other laboratory exams were done at the same time for the diagnosis of SAP. Receiver Operating Characteristic (ROC) curve analysis was performed to evaluate neutrophil CD64 as a biomarker of secondary infection in patients with SAP.
Results: Twenty-seven SAP patients were diagnosed with secondary infection 7 to 14 d after admission. ROC curve analysis showed that at a neutrophil CD64 index of 3.52, secondary infection identification exhibited high sensitivity (85.2%) and specificity (87.5%), and Youden index was 0.727.
Conclusions: The index of peripheral blood neutrophil CD64 is a highly sensitive marker for suspected secondary infection in SAP patients. Our study suggests that the neutrophil CD64 index could be a valuable marker for diagnosing infections subsequent to SAP.
Keywords
Neutrophil CD64 index, Severe acute pancreatitis, Secondary infection, Flow cytometry.
Introduction
Severe Acute Pancreatitis (SAP) is a critical clinical emergency. Once infection occurs in SAP, patients are highly susceptible to Multiple Organ Dysfunction Syndrome (MODS) and Multiple Organ Failure (MOF). About 80% of SAP patients die of complications caused by secondary infection [1,2]. A previous carefully designed, large-sample study showed that prophylactic administration of intravenous antibiotics does not reduce the incidence of infection subsequent to SAP [3].
It is critically important to diagnose secondary bacterial infection in time for effective treatment. At present, bacterial and other infectious diseases are confirmed using microbiological culture methods. However, microbiological tests are time consuming, and the results are often negative in patients who are receiving antibiotics. Some traditional laboratory markers, such as White Blood Cell (WBC) count and levels of C-Reactive Protein (CRP) and Procalcitonin (PCT), exhibit low specificity and limited correlation with bacterial infections. CRP and Procalcitonin have ever been thought more reliable test for sepsis. PCT is more widely used in the diagnosis of severe sepsis and bacterial infections. They are also used in monitoring the success of antimicrobial treatment. However, they might as well increase in non-infectious conditions too, such as severe congestive heart failure and acute pancreatitis [4-6].
Emerging evidence indicates that neutrophil CD64 (nCD64) is a highly sensitive and specific marker for systemic bacterial infection [7]. The most significant feature of CD64 is that its expression on neutrophils is not induced by non-infectious diseases or viral infection. Several studies have suggested that nCD64 expression is a useful biomarker in the management of hospital patients with bacterial infections [8,9]. Accordingly, we conducted a prospective study involving forty-three SAP patients, to investigate the value of the nCD64 index for diagnosing secondary infection in SAP patients.
Materials and Methods
Patients and setting
This study was conducted in the Department of Gastroenterology at the Second People’s Hospital of Nantong City, China, between January 2011 and December 2015. Forty-three patients admitted to the Gastroenterology Intensive Care Unit (GICU) with clinical diagnosis of SAP were enrolled in the study. Patients exhibiting respiratory, urinary tract, or skin infections were excluded. The study was approved by the Research Ethics Committee of the Second People’s Hospital of Nantong City. Written informed consent was received from all patients enrolled or from their guardians. The study group included 23 men and 20 women ranging from 46 to 87 y of age, with an average age of 56.24 ± 12.35 y. The Acute Physiology and Chronic Health Evaluation (APACHE II) and Rason average scores for the study group were 13.5 ± 2.8 and 4.6 ± 1.2, respectively.
Blood collection and analysis
Blood samples were collected from all patients in the study group from d 7 to 14 following admission to the GICU. Venous blood was processed for the determination of WBC count and CRP levels. Blood samples were also simultaneously cultured for microorganisms. Seven days after treatment of antiinfection, nCD64 index and CRP levels in the infectious group were measured again, and blood culture was also conducted for comparison. Lymphocytes were used as an internal reference to evaluate nCD64 expression levels in order to develop a novel method for diagnosing secondary bacterial infection in SAP patients. Flow Cytometry (FCM) was used to detect changes in the nCD64 index 7 to 14 d after the onset of SAP.
FCM
Blood samples were stained with CD64-FITC (Beckman Coulter), according to the manufacturer’s instructions. As CD64 is expressed on neutrophils and lymphocytes, the analyses were performed using lymphocytes and monocytes, with lymphocytes serving as an internal negative control. The ratio of average fluorescence intensity of nCD64 to average fluorescence intensity of lymphocyte CD64 was defined as the nCD64 index. A Beckman Coulter FC500 instrument was used for FCM.
Diagnostic criteria of secondary infection subsequent to SAP
The diagnosis of secondary infection subsequent to SAP was confirmed by blood culture, with patients divided into ‘positive’ and ‘negative’ groups based on these results. Receiver Operating Characteristic (ROC) curves were used to analyse and define the diagnostic cut-off value. The area under the ROC curve was calculated to quantify the predictive value of the nCD64 index. The sensitivityspecificity and Youden index at the optimum cut-off of patients’ nCD64 ratios were also calculated.
Statistical analyses
The Statistical Package for the Social Sciences (SPSS), version 20.0 (SPSS, Chicago, IL, USA) was used for statistical analyses. The ratio of nCD64 to lymphocyte CD64 fluorescence was calculated first from median fluorescence intensities and then transformed to (or very nearly to) a Gaussian distribution by logarithmic transformation. Differences between the two groups were analysed using a t-test for transformed data. The optimum cut-off point of the nCD64 index was determined by analysis of ROC curves.
Results
Changes of nCD64 Index, CRP concentration, and WBC count in SAP patients
At GCU admission, the average nCD64 index of the secondary infection patients was significantly higher than that of patients without secondary infection (8.64 ± 4.79 vs. 2.57 ± 1.07, P<0.05 by t-test). The mean CRP level was 136 ± 12.83 mg/L in infectious group and it was 129 ± 15.21 mg/L in non-infectious group. The mean WBC count was 15.61 ± 3.82 × 109/L in infectious group and it was 17.21 ± 2.92 × 109/L in non-infectious group. The mean CRP levels and WBC count did not differ significantly between the two groups. Expression of nCD64 was higher in SAP patients with positive blood cultures than in those with negative cultures (Table 1). Expression of nCD64 was similar between patients infected with gram-positive bacteria and those infected with gram-negative bacteria (8.65 ± 4.85 vs. 8.59 ± 4.67; P>0.05 by t-test).
| n | nCD64 index | WBC (× 109/L) | CRP (mg/L) | |
|---|---|---|---|---|
| Infectious group | 27 | (8.64 ± 4.79)* | (15.6 ± 3.82) | (136 ± 12.83) |
| Non-infectious group | 16 | (2.57 ± 1.07) | (17.22 ± 2.92) | (129 ± 15.21) |
| Notes: Data are presented as mean ± sd. t-test between two groups (*P<0.05). nCD64 index: Neutrophil CD64 index; WBC: White Blood Cell; CRP: C-Reactive Protein. | ||||
Table 1. The patient laboratory variables at the time of study inclusion.
Changes of nCD64 index before and after treatment in SAP patients acquiring secondary infection
Blood culture results are regarded as the gold standard for the diagnosis of infection subsequent to SAP. In the present study, a total of 27 SAP patients were diagnosed with secondary infection based on blood culture results. A total of 16 SAP patients did not develop secondary infection. After anti-infection treatment, the mean nCD64 index of infection group was 3.35 ± 1.28 in the infectious group, mean CRP level was 56 ± 7.62 mg/ml and mean WBC count was 6.64 ± 1.35 × 109/L. The mean nCD64 index, CRP levels and WBC count differed significantly before and after treatment in the infectious group (Table 2).
| n | nCD64 index | WBC (× 109/L) | CRP (mg/L) | |
|---|---|---|---|---|
| Before treatment | 27 | (8.64 ± 4.79)* | (15.6 ± 3.82) | (136 ± 12.83) |
| After treatment | 27 | (3.35 ± 1.28) | (6.64 ± 1.35) | (56 ± 7.62) |
| Notes: Data are presented as mean ± sd. t-test between two groups (*P<0.05). nCD64 index: Neutrophil CD64 index; WBC: White Blood Cell; CRP:C-Reactive Protein. | ||||
Table 2. The Patient laboratory variables before and after treatment in the infectious group.
Value of the nCD64 index for diagnosing infection subsequent to SAP
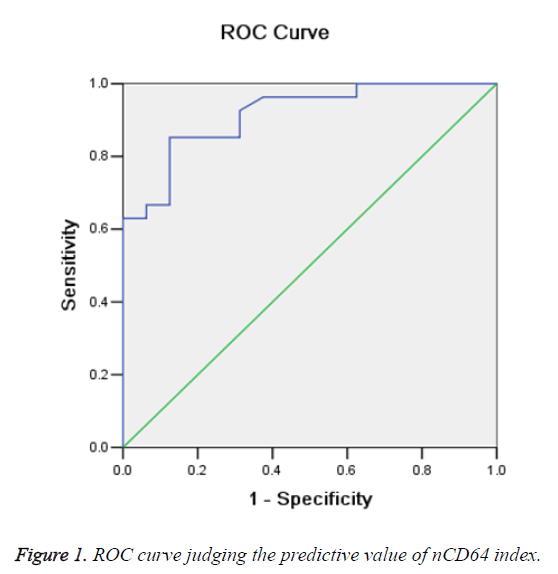
ROC curves were constructed to examine the sensitivity and specificity of the nCD64 index in distinguishing patients with secondary infection from those without (Figure 1). The area under the ROC curve was 0.916 (95% confidence interval, 0.834-0.997) (Table 2).
Based on the Youden index, the optimal cut-off point of the nCD64 index was determined as 3.52. According to ROC curve analyses, at the optimum cut-off point, the nCD64 index would exhibit a sensitivity of 85.2%, specificity of 87.5%, and Youden index of 0.727. The results of microbial culture were negative in 17 patients with an nCD64 index<3.52 and positive in 26 patients with an nCD64 index>3.52.
There occurred one false negative case based on the results of bacterial culture. These results indicate that the nCD64 index is a useful biomarker for the diagnosis of secondary bacterial infection in SAP patients.
Time required for diagnosis of secondary infection using the nCD64 index
Traditional blood culture tests indicated secondary infection in 27 SAP patients. It took an average of 48 ± 15 h to obtain results of culture tests and an average of 106 ± 22 h to identify the organisms, whereas FCM-based nCD64 results were available in 12 ± 8 h. Diagnosis of secondary infection by determination of the nCD64 index using FCM thus required significantly less time than traditional blood culture testing (P<0.05 by t-test).
Discussion
The pathogenesis of SAP can be generally divided into three stages. The first stage (also known as the “acute phase”) is characterized by the occurrence of the Systemic Inflammatory Response Syndrome (SIRS), MODS, or even MOF. This stage usually lasts from 1 to 2 w. Mortality in the first stage typically results from MODS or MOF. The second stage is known as the evolutional phase. The most characteristic feature of this phase is necrosis of the pancreas. Local complications include acute peripancreatic fluid accumulation, necrotic liquid accumulation, and encapsulated necrosis. In this phase, the necrotic foci are usually sterile but can easily become infected. Four weeks after onset, patients enter the late stage (also known as the infectious phase), in which secondary bacterial and deep fungal infections are more likely to occur due to necrosis of pancreatic tissue. Secondary infection is the primary cause of death in SAP. How to diagnose and cure the secondary infection in time is the most important problem for clinicians [9,10].
Translocation of bacteria from the intestine represents the primary source of secondary infection in SAP patients. During SAP, bacteria that permanently reside in the intestinal tract can translocate and release large amounts of antitoxins that can penetrate the intestinal mucosa and enter the blood, leading to bacteremia and secondary infection. Bacteremia and endotoxemia can aggravate SIRS, inducing the onset of MODS and MOF. The abuse of broad-spectrum antibiotics has dramatically increased the number of resistant bacterial strains, thus increasing the chances of double infection and the economic burden on patients, prolonging the length of hospital stay, and increasing the SAP mortality rate [11,12].
During the early stages of SAP, such as the acute phase or the evolutional phase, the focus of infection is typically too small to be detected using imaging examinations such as ultrasound, X-ray computed tomography, and magnetic resonance imaging. Blood culture and subsequent strain identification, therefore, remain the gold standard for diagnosing bacteremia in SAP. However, obtaining accurate results with blood culture tests is usually time-consuming. The average course of blood culture testing is 3 to 5 d, and some pathogenic bacteria require 7 d or longer to culture. At present, the diagnosis and prognosis of secondary infection in patients with SAP are commonly based on the concentrations of serum CRP and PCT. However, further research is needed to verify the clinical practical value of CRP and PCT [13,14].
CD64, or immunoglobulin Fc-γ receptor I, is expressed on monocytes and neutrophils. In the absence of stimulation or in the non-infected state, CD64 is expressed at very low levels. Once neutrophils are exposed to bacteria or bacterial endotoxins, the neutrophil respiratory burst activity is enhanced and the expression of CD64 is significantly up-regulated. Thus, CD64 is commonly used as a marker of neutrophil activation [4,15,16]. Studies have indicated that nCD64 is a highly sensitive and specific marker for diagnosing systemic infection and sepsis [17], and thus its expression is often used to diagnose bacterial infection and evaluate disease severity.
The present study evaluated whether nCD64 expression is useful for the early diagnosis of secondary infection in patients with SAP. In contrast to previously published studies involving larger samples of heterogeneous cohorts, including medical, surgical, and trauma patients [18], this study focused on secondary infection in a relatively small number of SAP patients. Cultures of blood/exudate were positive in 27 patients 7 to 14 d after onset and negative in 16 patients. We found that the nCD64 index exhibited a sensitivity of 85.2% and specificity of 87.5% for diagnosing secondary infection subsequent to SAP and Youden index was calculated as 0.727. The 85.2% sensitivity and 87.5% specificity determined in this study are in line with previous studies involving critically ill patients [19,20].
The inclusion of only patients with microbiologically confirmed infection would lead to a higher cut-off and higher nCD64 expression [21,22]. Other research indicated that nCD64 expression has greater diagnostic accuracy in discriminating septic from nonseptic patients than in discriminating bacterial from non-bacterial infections [23]. In this study, the significant correlation between nCD64 and severity of SAP and patient prognosis, suggest that nCD64 is significantly better than the markers of infection used currently. The nCD64 index, as determined by FCM, retains the sensitivity of conventional blood culture and peritoneal fluid culture methods and maintains a high positive predictive value. The present results indicate that the nCD64 index is useful for the diagnosis of secondary infection in SAP. A low nCD64 index allows clinicians to confidently exclude secondary infection in SAP patients; conversely, an index value above the cut-off therefore provides a strong indication of secondary infection in SAP patients, enhancing the likelihood of appropriate treatment.
In this study, it took an average of 48 ± 15 h to obtain results of culture tests and an average of 106 ± 22 h to identify the organisms causing the infections. In contrast, only 12 ± 8 h were needed to determine the nCD64 index using FCM analysis; FCM analysis required significantly less time than traditional culture testing (P<0.05). The pretreatment of SAP patients with antibiotics can significantly affect both the time required and reliability of culture methods using peripheral blood. To a certain degree, reducing the use of antibiotics can reduce the incidence of super infections, as well as mortality, thus shortening the hospital length of stay. The use of the nCD64 index to guide antibiotic therapy can eliminate unnecessary antibiotic administration by significantly shortening the duration of treatment in patients with SAP, minimizing the risks of both bacterial drug resistance development and super infection.
The main limitation of our study is the number of patients included. We recommend performing more studies with a higher number of patients. By this way it will be possible to obtain more powerful data on using CD64 and predicting bacteremia.
Conclusion
Determination of the nCD64 index using FCM is a superior diagnostic technology because the nCD64 index presents unique advantages and high practical value for the differential diagnosis of infection. Due to its high sensitivity and relatively short determination time, the nCD64 index is a particularly useful biomarker for diagnosing bacterial infections in SAP patients, and is therefore worthy of further promotion.
Acknowledgments
This study was supported by the Nantong Municipal Commission of Science and Technology (qyz15059)).
References
- Feng C, Li F. Rediscussion on severe acute pancreatitis (SAP) infection and reasonable application of prophylactic antibiotics. J Hepatopancreatobil Surg 2011; 23: 77-83.
- Guo ZH, Hao JY. Rational use of antibiotics in patients with severe acute pancreatitis. Chin J Diges 2012; 32: 585-587.
- Wittau M, Mayer B, Scheele J, Henne-Bruns D, Dellinger EP, Isenmann R. Systematic review and meta-analysis of antibiotic prophylaxis in severe acute pancreatitis. Scand J Gastroenterol 2011; 46: 261-270.
- Cid J, Aguinaco R, Sanchez R, Garcia-Pardo G, Llorente A. Neutrophil CD64 expression as marker of bacterial infection: a systematic review and meta-analysis. J Infect 2010; 60: 313-319.
- Koeze J, Hendrix MG, van den Bergh FA, Brouwer RM, Zijlstra JG. In critically ill patients the procalcitonin level can be misleading. Crit Care 2011; 15: 422.
- Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis 2013; 13: 426-435.
- Wan DP, Liu DY. The value of spiral CT scan in the diagnosis of severe acute pancreatitis complicated with infection. J Clin Med Test 2014; 11: 2000-2001.
- Oppegaard O, Skodvin B, Halse AK, Langeland N. CD64 as a potential biomarker in septic arthritis. BMC Infect Dis 2013; 13: 278.
- Yu QQ, Wang HP, Zhai ZM, Zhao YM. Clinical significance of determination of neutrophil CD64 mean fluorescence intensity index using flow cytometry in diagnosis of infection diseases. Clin Focus 2011; 26: 383-387.
- Yin T, Wang CY. Key points and difficulties of multidisciplinary comprehensive treatment for severe acute pancreatitis. Chin J Gen Surg 2015; 30: 1-3.
- De Waele JJ. Rational use of antimicrobials in patients with severe acute pancreatitis. Semin Respir Crit Care Med 2011; 32: 174-180.
- Jiang K, Huang W, Yang XN, Xia Q. Present and future of prophylactic antibiotics for severe acute pancreatitis. World J Gastroenterol 2012; 18: 279-284.
- Ding KH, Ban FZ, Huang CL, Li RN, Huang HH. Clinical significance and changes on serum levels of PCT, hs-CRP, IL-6 in patients with acute pancreatitis. Int J Lab Med 2013; 34: 2241-2242.
- Lai XR, Tong HS, Lin HJ, Fu YJ, He MR. Detection and significance of serum calcitonin level in patients with acute pancreatitis. Guangdong Med J 2013; 34: 2224-2226.
- El Shimi MS, Abou Shady NM, Hamed GM, Shedeed NS. Significance of neutrophilic CD64 as an early marker for detection of neonatal sepsis and prediction of disease outcome. J Matern Fetal Neonatal Med 2017; 30: 1709-1714.
- van der Meer W, Pickkers P, Scott CS, van der Hoeven JG, Gunnewiek JK. Hematological indices, inflammatory markers and neutrophil CD64 expression: comparative trends during experimental human endotoxemia. J Endotoxin Res 2007; 13: 94-100.
- Groselj-Grenc M, Ihan A, Pavcnik-Arnol M, Kopitar AN, Gmeiner-Stopar T, Derganc M. Neutrophil and monocyte CD64 indexes, lipopolysaccharide-binding protein, procalcitonin and C-reactive protein in sepsis of critically ill neonates and children. Intensive Care Med 2009; 35: 1950-1958.
- Mokuda S, Doi O, Takasugi K. Simultaneous quantitative analysis of the expression of CD64 and CD35 on neutrophils as markers to differentiate between bacterial and viral infections in patients with rheumatoid arthritis. Mod Rheumatol 2012; 22: 750-757.
- Gibot S, Bene MC, Noel R, Massin F, Guy J, Cravoisy A, Barraud D, De Carvalho Bittencourt M, Quenot JP, Bollaert PE, Faure G, Charles PE. Combination biomarkers to diagnose sepsis in the critically ill patient. Am J Respir Crit Care Med 2012; 186: 65-71.
- Hsu KH, Chan MC, Wang JM, Lin LY, Wu CL. Comparison of Fcgamma receptor expression on neutrophils with procalcitonin for the diagnosis of sepsis in critically ill patients. Respirology 2011; 16: 152-160.
- Dilli D, Oguz SS, Dilmen U, Koker MY, Kizilgun M. Predictive values of neutrophil CD64 expression compared with interleukin-6 and C-reactive protein in early diagnosis of neonatal sepsis. J Clin Lab Anal 2010; 24: 363-370.
- Nuutila J. The novel applications of the quantitative analysis of neutrophil cell surface FcgammaRI (CD64) to the diagnosis of infectious and inflammatory diseases. Curr Opin Infect Dis 2010; 23: 268-274.
- Gros A, Roussel M, Sauvadet E, Gacouin A, Marque S, Chimot L, Lavoue S, Camus C, Fest T, Le Tulzo Y. The sensitivity of neutrophil CD64 expression as a biomarker of bacterial infection is low in critically ill patients. Intensive Care Med 2012; 38: 445-452.