Research Article - Journal of Molecular Oncology Research (2022) Volume 6, Issue 6
Validity and impact of COX-2 "Cyclooxygenase-2" in breast cancer.
Fatma Z Abd Elrahman1*, Adel Gabr1, Amen H Zaky1, Ashraf Zedan1, Tarek M Elsaba2
1Department of Oncology and Hematology, Assiut University, Asyut, Egypt
2Department of Pathology, Assiut University, Asyut, Egypt
- Corresponding Author:
- Dr. Fatma Z Abd Elrahman
Department of Oncology and Hematology
Assiut University
Asyut
Egypt
E-mail: fatma_zakaria_86@yahoo.com
Received: 15-February-2021, Manuscript No. M-26181; Editor assigned: 18-February-2021, PreQC No. P-26181; Reviewed: 04-March-2022, QC No. Q-26181; Revised: 31-May-2022, QI No. Q-26181; Manuscript No. R-26181; Published: 28-June-2022, DOI: 10.35841/aamor-6.6.131
Citation: Fatma Z Abd Elrahman, Adel Gabr, Amen H Zaky, et al.. Validity and impact of COX-2 “Cyclooxygenase-2” in breast cancer. J Mol Oncol 2022;6(6):1-6.
Abstract
Background: Breast carcinoma is the most common malignant tumor and the leading cause of carcinoma deaths in women. Its etiology is multifactorial, including reproductive factors, hormonal imbalances and genetic predispositions. According to many studies Cycloxygenase-2 (COX-2) plays an important role in the carcinogenesis and increased expression has been regarded as a poor prognostic factor.
Objective: The objective of our study is to evaluate COX-2 expression in breast cancer comparing two different scoring system.
Methods: Formalin-fixed and paraffin-embedded tissue blocks were studied for COX-2 expression by immunohistochemistry in 100 patients diagnosed as breast carcinoma. Two different scoring system were applied. The relationship between COX-2 expression and various clinico-pathological parameters was studied.
Results: The results of our study suggest an association of the expression of COX-2 to the poor prognostic factors in breast cancer, such as larger tumor size, positive lymph node status, higher T stage and N stage, hormonal status and HER-2/NEU status as studying the association between COX-2 protein expression and different clinco-pathologic features revealed that larger tumor size (>5) and lymph node metastasis showed statistical significant association with COX2 protein expression (p=0.014 and p=0.031, respectively). While rest of clinico-pathologic features such as age, stage, hormonal receptor status and histopathologic features showed no statistical significant association. However, Studying the association between COX-2 protein expression using H-score and clinico-pathological characteristics revealed that The median H-score of COX-2 protein expression was higher in her-2/neu positive cases compared to her2/neu negative cases and that was statistically significant with a p value (p=0.023). Also, statistical significant association was found between hormonal receptor status and median H-score of COX-2 protein expression (p=0.029).
Conclusion: Applying different scoring system resulted in different significant data. Therefore, standardized scoring system for COX-2 protein expression should be developed.
Keywords
COX-2, Breast, Prognosi, Clinico-pathologic, Carcinoma.
Introduction
Breast cancer is the most frequently diagnosed cancer globally and is the leading cause of cancer-related death in women [1]. In 2018, the predicted number of new breast cancers in 28 European Union (EU) countries was 404,920 with estimated age-adjusted annual incidence of breast cancer of 144.9/100000 and mortality of 32.9/100000, with 98,755 predicted deaths. In Egypt, it is the most common cancer in females, in 2018 the incidence of breast cancer was 23081 new cases about 35.1% of the incidence of all cancer cases according to Globocan 2018. A female breast cancer is a challenging health problem coming on top of all malignancies [2] with a poor outcome compared to international figures [3]. Many studies showed that age at diagnosis of breast cancer in Arab countries is a decade younger than that in Western countries [4].
In breast cancer the molecular characteristics play an important role in tumor prognosis and aggressiveness and may contribute to routine clinical decision making. Additionally, identifying specific molecular patterns help to introduce targeted therapies for cancer treatment. The classical molecular prognostic parameters of breast cancer are Estrogen Receptor (ER), Progesterone Receptor (PR) expression and Her-2-neu receptor expression [5,6]. Studies have shown that Cycloxygenase-2 (COX-2) plays an important role in the development of some human cancers, specifically pulmonary, colon and breast cancers. Cyclooxygenase enhances catalyzing the conversion of arachidonic acid to prostaglandin endoperoxide, which is the rate limiting step in prostaglandin and thromboxane biosynthesis. COX-1 and COX-2 are the two isoforms of prostaglandin synthase [7].
COX-1 is characterized as a housekeeping enzyme required for the maintenance of basal level prostaglandins and is expressed constitutively in most tissues. COX-2 is highly inducible and can be rapidly up regulated in response to various proinflammatory agents, including cytokines, mitogens, and tumor promoters, especially in cells involved in inflammation, pain, fever, Alzheimer's disease, osteoarthritis, or tumor formation [8,9].
Under normal conditions, acute inflammation is a tightly controlled self-limiting response, specific cytokines, including interleukin-1 (IL-1) and IL-6, exert feedback inhibition causing COX-2 expression and PGE2 production to cease and the inflammatory response to subside. However, with sustained exposure to pro inflammatory stimuli, continued expression of COX-2 leads to the transition from acute to chronic inflammation. Moreover, COX-2 plays a role in the regulation of estrogen by producing prostaglandin E2, which increases the expression of the cytochrome P450 enzyme complex (also known as aromatase) that catalyzes androgen to produce estrogen [10-12]. During progression of cancer, prostaglandins mediate several mechanisms, including cell proliferation, apoptosis, and angiogenesis. Therefore, the aim of our study is to evaluate the COX-2 protein expression in breast cancer and its relation with clinical and histological prognostic parameters applying two different scoring systems for interpretation and reporting of immunohistochemistry results and comparing them.
Materials and Methods
A total number of one hundred formalin-fixed and paraffin-embedded tissue blocks were collected from the archived materials of pathology department in the South Egypt Cancer Institute. There were taken either by True cut biopsy, breast conservative surgery or modified radical mastectomy. Clinicopathological parameters such as patient age, gender, Tumor size (T), Lymph Node metastasis (LN) hormonal status (ER and PR), HER-2/NEU and stage, all were obtained from the available histopathological reports, and the overall survival was obtained from the patient medical record files of SECI.
Immunohistochemistry
Three μm thick formalin-fixed paraffin-embedded tissue sections were cut and Sections were dewaxed in Xylene (for half an hour) and rehydrated through graded alcohols from 100%-70% then washed in Distilled water. Pre-treatment with Heat-Induced Epitope Retrieval (HIER) was done using citrate buffer pH 9 for 20 minutes at 97°C. Slides were then washed 2-3 times with Phosphate Buffer Solution (PBS). Blocking of endogenous peroxidase activity was performed using peroxidase blocking reagent (Genemed, Sakura, USA) and incubated 5 minutes a Polyclonal Anti-PTGS2/COX-2 antibody with Catalog no. #YPA1044 primary antibody (Chongqing Biospes Co., Ltd, China) diluted by 1:150 was applied to the sections and incubated for 30 minutes at room temperature. Then the slides were washed 2-3 times using PBS. After washing, immunostaining was performed using a universal staining kit, (Poly HRP/DAB (Ready-To-Use), Genemed, Sakura, USA) following the manufacturer’s instructions. The secondary antibody was applied to the slides and incubated for 20 minutes at room temperature, then rinsed and washed with PBS twice, the detection was done by DAB chromogen and substrate for 5 min using the same kit. Sections were then counterstained using Mayer’s hematoxylin (Dako, Denmark) for 5 minutes then washed in distilled water, dehydrated in ascending alcohols from 70%-100% then cleared in Xylene and left to dry in air room temperature in a humidity chamber to prevent unnecessary background staining.
Evaluation of COX-2 protein expression
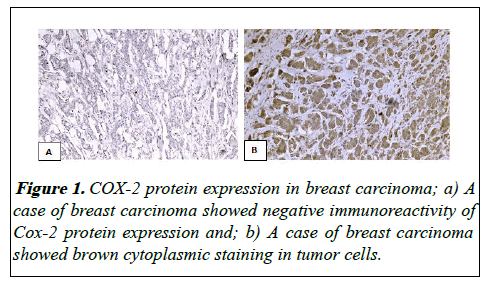
COX-2 positivity was indicated by the presence of brown cytoplasmic staining. Two different approaches were applied for evaluation of COX-2 protein expression in breast tissue. The first scoring system was categorizing COX-2 protein expression into negative (no stained cells) and positive [13,14]. In the second approach, Staining was assessed using H-score, which is a semi-quantitative approach. In this approach, staining intensity was first determined for all cells (0, 1, 2, 3, for negative, weak, moderate and strong intensity respectively), then the percentage of cells at each staining intensity was calculated and finally H-score is calculated using the following formula: (3 × percentage of strongly staining malignant cells)+(2 × percentage of moderately staining malignant cells)+(1 × percentage of weakly staining malignant cells) which give a range from 0 to 300 (Figure 1) [15].
Statistical analysis
All statistical calculations were done using SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) version 22. Data which are normally distributed were statistically described in terms of mean ± standard deviation ( ± SD), frequencies (number of cases) and percentages were used for qualitative data. For comparing quantitative data, Mann Whitney U test was performed because the data were not normally distributed. For comparing categorical data, Chi square (χ2) test was performed. Exact test was used instead when the expected frequency is less than 5. Kaplan-Meier test was performed to compare overall survival between both study groups. P-value is always 2 tailed set significant at 0.05 level.
Results
Clinic-pathological features
The mean age of our patients was 50 (50.82 ± 12.69) years. According to the stage 5% of cases were of stage I, 42% were of of stage II, 46% were stage III, and 8% were of stage IV. Regarding the tumor size, T2 was the commonest tumor size representing (50%) of cases followed by T3 (32%), T1 (13%) and T4 (5%) of cases The majority of cases presented by invasive ductal carcinoma by 95 %, only 5% were other histo-pathological types Regarding the hormonal profile; 69 cases were estrogen receptor positive. Also 63 cases were progesterone receptor positive, and 12 cases were Her-2/ NEU positive. All clinico-pathologic features are summarized in Table 1.
| Variable name | COX-2 status | p-value | ||
|---|---|---|---|---|
| Negative (n=24) | Positive (n=76) | |||
| N (%) | N (%) | |||
| Mean ± SD | 48.54 ± 17.73 | 51.54 ± 10.68 | 0.316 | |
| Age (years) | ≤ 50 | 16 (66.7) | 37 (48.7) | 0.124 |
| ?50 | 8 (33.3) | 39 (51.3) | ||
| Site of tumor | Right | 14 (58.3) | 44 (57.9) | 0.97 |
| Left | 10 (41.7) | 32 (42.1) | ||
| Stage | Early | 13 (54.2) | 33 (43.4) | 0.357 |
| Advanced | 11 (45.8) | 43 (56.6) | ||
| Tumor size (cm) | ≤ 5 | 20 (83.3) | 42 (55.3) | 0.014* |
| ?5 | 4 (16.7) | 34 (44.7) | ||
| Lymph node metastasis | negative | 10 (41.7) | 15 (19.7) | 0.031* |
| positive | 14 (58.3) | 61 (80.3) | ||
| Hormonal receptors | Negative | 7 (29.2) | 17 (22.4) | 0.497 |
| Positive | 17 (70.8) | 59 (77.6) | ||
| HER2/neu | Negative | 21 (87.5) | 59 (77.6) | 0.387 |
| Positive | 3 (12.5) | 17 (22.4) | ||
| Pathology | IDC | 22 (91.7) | 73 (96.1) | 0.591 |
| Other pathology | 2 (8.3) | 3 (3.9) | ||
Data are mean ± SD or n (%)* Significance defined by p<0.05.
Table 1: Phytoconstituents in Morinda citrifolia L.
Association of COX2 protein expression (positive vs negative) and different clinic-pathologic features
Evaluation of COX-2 protein expression as positive en negative expression revealed that 76% of cases were positive for COX-2 protein expression.
Studying the association between COX-2 protein expression and different clinco-pathologic features revealed that larger tumor size (>5) and lymph node metastasis showed statistical significant association with COX-2 protein expression (p=0.014 and p=0.031, respectively). While rest of clinico-pathologic features such as age, stage, hormonal receptor status and histopathologic features showed no statistical significant association (Tables 2 and 3).
| Variable name | N=100 | |
|---|---|---|
| N (%) | ||
| Age (years), mean ± SD | 50.82 ± 12.69 | |
| Gender | Male | 1 (1.0) |
| Female | 99 (99.0) | |
| Site of tumor | Right | 58 (58.0) |
| Left | 42 (42.0) | |
| Stage | Stage 1 | 5 (5.0) |
| Stage 2 | 41 (41.0) | |
| Stage 3 | 46 (46.0) | |
| Stage 4 | 8 (8.0) | |
| Tumor size | T1 | 13 (13.0) |
| T2 | 50 (50.0) | |
| T3 | 32 (32.0) | |
| T4 | 5 (5.0) | |
| Lymph node metastasis | N0 | 25 (25.0) |
| N1 | 26 (26.0) | |
| N2 | 19 (19.0) | |
| N3 | 30 (30.0) | |
| ER | Negative | 31 (31.0) |
| Positive | 69 (69.0) | |
| PR | Negative | 37 (37.0) |
| Positive | 63 (63.0) | |
| HER2/neu | Negative | 80 (80.0) |
| Positive | 20 (20.0) | |
| Pathology | IDC | 95 (95.0) |
| Other Pathology | 5 (5.0) | |
Table 2: Clinico-pathological details of all study participants.
| Variable name | H score | p-value | |
|---|---|---|---|
| Median range) | |||
| Age | ≤ 50 | 200 (30-300) | 0.191 |
| >50 | 200 (10-300) | ||
| Site of tumor | Right | 200 (10-300) | 0.466 |
| Left | 200 (60-300) | ||
| Stage | Early | 200 (30-300) | 0.236 |
| Advanced | 200 (10-300) | ||
| Tumor size | <5 | 200 (20-300) | 0.331 |
| ≥ 5 | 200 (10-300) | ||
| Lymph node metastasis | negative | 200 (60-300) | 0.871 |
| positive | 200 (10-300) | ||
| Hormonal receptors | Negative | 200 (120-300) | 0.029* |
| Positive | 200 (10-300) | ||
| HER-2/neu | Negative | 200 (20-300) | 0.023* |
| Positive | 300 (10-300) | ||
Table 3: Association of COX2 protein expression using H-score and different clinico-pathologic features.
Association between COX-2 protein expression and different clinico-pathologic features using H-score
Studying the association between COX-2 protein expression using H-score and clinico-pathological characteristics revealed that the median H-score of COX-2 protein expression was higher in her2/neu positive cases compared to her2/neu negative cases and that was statistically significant with a p value (p=0.023). Also, statistical significant association was found between hormonal receptor status and median H-score of COX-2 protein expression (p=0.029). No statistical significant association was found between median H-score of COX-2 protein expression and age (p=0.19), site of tumor (p=0.466), stage (p=0.236), tumor size (0.331), and lymph node metastasis (p=0.871) (Tables 4 and 5).
| Survival | Estimate ± SE | P-value | |
|---|---|---|---|
| Negative | Positive | ||
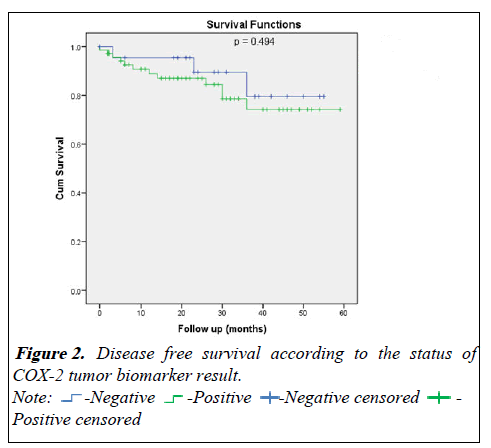
| At 1 year | 95.5 ± 4.4% | 88.9 ± 4.0% | 0.494 |
| At 2 year | 89.5 ± 7.1% | 87.0 ± 4.3% | |
| At 3 year | 79.5 ± 11.3% | 74.2 ± 7.1% | |
| At 4 year | 79.5 ± 11.3% | 74.2 ± 7.1% | |
Table 4: Disease free survival according to the status of COX-2 tumor biomarker result.
| Survival | Estimate ± SE | P-value | |
|---|---|---|---|
| Negative | Positive | ||
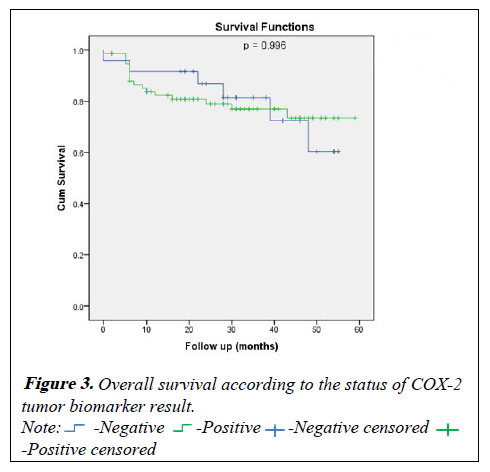
| At 1 year | 91.7 ± 5.6% | 82.3 ± 4.5% | 0.996 |
| At 2 year | 86.8 ± 7.1% | 79.0 ± 4.9% | |
| At 3 year | 81.4 ± 8.5% | 76.9 ± 5.2% | |
| At 4 year | 60.3 ± 14.5% | 73.4 ± 6.0% | |
Table 5: Overall survival according to the status of COX-2 tumor biomarker result.
Survival analysis
For the disease free survival analysis and overall survival are shown using Kaplan-Meier survival curves, that wasn't show any significance between COX positive or negative (p=0.494) and (p=0.996) respectively (Figures 2 and 3).
Discussion
In our study we evaluated COX-2 protein expression by two different scoring system. According to COX-2 positive or negative, 76% of the studied breast carcinoma cases showed COX-2 positivity. This finding is comparable with the findings of various studies [16,17].
COX-2 protein expression was statistically significantly correlated with large size tumors in our study [18].
COX- 2 expression to be more frequent in patients with lymph node metastasis, these findings were in concordance with the studies done [20]. However, there was no significant correlation between COX-2 positivity and node status. Correlation between lymph node positivity and higher COX-2 expression is associated with tumor spread and a poor prognosis [21,22]. Regarding the other factors, including age, hormonal status, stage of tumor, and HER-2/NEU status there is no significant by cox positivity and negativity, these data is different to many studies that demonstrated that COX-2 expression was significantly correlated with advanced stage of disease, hormonal negativity and HER-2/NEU status findings were observed [23,24].
Regarding calculating COX-2 by H-score in our study, it showed significance association with, negative hormonal status, and positive HER-2/NEU which is similar to various studies reported that COX-2 expression was correlated with ER negative PR negative and HER-2/neu positive status which may be explained as COX-2 expression in ER negative cell lines is also associated with mutated RAS. Increased expression of this protein has been associated with reduced estrogen dependence in breast cells [25-27]. Both PKC and mutated RAS have been associated with an increased metastatic potential in cell lines [28,29].
HER-2/neu is over expressed in approximately 20%-30% of invasive breast cancers and is an independent marker of poor prognosis [30]. We found that high levels of COX-2 expression correlated with HER-2⁄neu overexpression which show highly significant, which explained by COX-2 can stimulate HER-2/neu expression via EGFR through PGE-2. So COX-2 mediates variety of cellular processes including tumor growth, apoptosis, differentiation, cell cycle, lymph node metastasis and angiogenesis, however no significant correlation was found between COX-2 status and estrogen receptor status (P value=0.74), progesterone receptor status (P value=0.91) or HER-2-neu expression (P value=0.74) [31].
Conclusion
To sum up, the aforementioned data showed that applying two different scoring systems for evaluation and interpretation of COX-2 protein expression resulted in different significant data which make comparison of the results of different studies difficult and the resulting data are not robust enough to draw a conclusion regarding COX-2 as a prognostic factor. Therefore, further investigations should develop anew standards for evaluation of COX-2 expression and do not rely on researchers opinions to choose which scoring system to apply.
References
- Mohammad H, Forouzanfar KJF, Allyne M, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: A systematic analysis. Lancet. 2011;378998010:1461-84.
- Ferlay J, Shin HR, Bray F, et al. Cancer incidence and mortality worldwide. IARC Cancer: International agency for research on cancer. 2010.
- El Saghir NS, Seoud M, Khalil MK, et al. Effects of young age at presentation on survival in breast cancer. BMC Cancer. 2006;6:194.
- Pakkiri P, Lakhani SR, Smart CE. Current and future approach to the pathologist’s assessment for targeted therapy in breast cancer. Pathology. 2009;41(1):89-99.
- Ross JS, Slodkowska EA, Symmans WF, et al. The HER-2 receptor and breast cancer: Ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320-69.
- Williams CDR. Prostaglandin endoperoxide synthase: Why two isoforms? Am J Physiol Gastrointest Liver Physiol. 1996;270:393-400.
- Claria J. Cyclooxygenase-2 biology. Curr Pharm Des. 2003;9(27):2177-2190.
- Turini ME, Raymond NDB. Cyclooxygenase-2: A therapeutic target. Annu Rev Med 2002;53(1):35-57.
- Brueggemeier R, Richards JPT. Aromatase and cyclooxygenases: Enzymes in breast cancer. J Steroid Biochem Mol Biol. 2003;15:501-7.
- Diaz-Cruz E, Shapiro CBR. Cyclooxygenase inhibitors suppress aromatase expression and activity in breast cancer cells. J Clin Endocrinol Metab. 2005;90(5):2563-70.
- Richards J, Petrel T. Signaling pathways regulating aromatase and cyclooxygenases in normal and malignant breast cells. J Steroid Biochem Mol Biol. 2002;80:203-12.
- Hwang D, Scollard D, Byrne J, et al. Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cancer. J Natl Cancer Inst. 1998;90:455-60.
- Leo C, Faber S, Hentschel B, et al. The status of cyclooxygenase-2 expression in ductal carcinoma in situ lesions and invasive breast cancer correlates to cyclooxygenase-2 expression in normal breast tissue. Ann Diagn Pathol. 2006;10:327-32.
- Cass JD. Automated quantitative analysis of p53, cyclin D1, Ki67 and pERK expression in breast carcinoma does not differ from expert pathologist scoring and correlates with clinico-pathological characteristics. Cancers. 2012;4:725-42.
- Mosalpuria K, Hall C, Krishnamurthy S, et al. Cyclooxygenase-2 expression in non-metastatic triple-negative breast cancer patients. Mol Clin Oncol. 2014;2:845-50.
- Rozenowicz R, Santos R, Rodrigues F, et al. COX-2 and its association with prognostic factors and response to neoadjuvant chemotherapy in patients with breast cancer. Eur J Surg Oncol. 2012;38:803.
- Jana D, Sarkar DK, Ganguly S, et al. Role of Cyclooxygenase 2 (COX-2) in prognosis of breast cancer. Indian J Surg Oncol. 2014;5:59-65.
- Lee J, Bae J, Woo S, et al. Correlation between COX-2 expression and hormone receptors in invasive ductal breast cancer. J Korean Surg Soc. 2010;78:140.
- Dannenberg AJHL. The role of COX-2 in breast and cervical cancer. Prog Exp Tumor Res. 2003;37:90-106.
- Costa C, Soares R, Reis-Filho J, et al. Cyclo-oxygenase 2 expression is associated with angiogenesis and lymph node metastasis in human breast cancer. J Clin Pathol. 2002;55:429-34.
- Misron N, Looi LMN. Cyclooxygenase-2 expression in invasive breast carcinomas of no special type and correlation with pathological profiles suggest a role in tumorigenesis rather than cancer progression. Asian Pac J Cancer Prev. 2015;16:1553-8.
- Ristimaki A, Sivula A, Lundin JL. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632-5.
- Arun B. The role of COX-2 inhibition in breast cancer treatment and prevention. Semin Oncol. 2004;31:22-9.
- Denkert C, Winzer KJ, Muller BM, et al. Elevated expression of cyclooxygenase-2 is a negative prognostic factor for disease survival and overall survival in patients with breast cancer. Cancer. 2003;97:2978-87.
- Zeeneldin AA, Mohamed AM, Abdel HA, et al. Survival effects of cyclooxygenase-2 and 12- lypooxygenase in Egyptian women with operable breast cancer. Indian J Cancer. 2009;46:54-60.
- Welch DRWL. Genetic and epigenetic regulation of human breast cancer progression and metastasis. Endocr Relat Cancer. 1998;5:155-97.
- Kiley SC, Clark KJ, Goodnough M, et al. Protein kinase C delta involvement in mammary tumour cell metastasis. Cancer Res. 1999;59:3230-38.
- Gilhooly EM, Rose DP. The association between a mutated ras gene and cyclooxygenase-2 expression in human breast cancer cell lines. Int J Oncol. 1999;15:167-70.
- Tsutsui S, Ohno S, Murakami S, et al. Prognostic value of c-erbB2 expression in breast cancer. J Surg Oncol. 2002;79:216-23.
- Solanki R, Agrawal N, Ansari M, et al. COX-2 expression in breast carcinoma with correlation to clinicopathological parameters. Asian Pacific J Cancer Prev. 2018;19:1971-75.
- Ristimaki A, Sivula A, Lundin J, et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632-35.
 Positive censored.
Positive censored.