Research Article - Journal of Industrial and Environmental Chemistry (2017) Volume 1, Issue 1
Utility of dispersive liquid-liquid microextraction based on ionic liquid for spectrophotometric determination of titanium in environmental samples
Alaa S Amin1*, Zakia Al-Malah21Chemistry Department, Benha University, Benha, Egypt
2Chemistry Department, Umm AL-Qura University, Makkah, Saudi Arabia
- *Corresponding Author:
- Alaa S Amin
Chemistry Department
Benha University
Benha
Egypt
Tel: +20552350996
E-mail: asamin2005@hotmail.com
Accepted Date: October 14, 2017
Citation: Amin AS, Al-Malah Z. Utility of dispersive liquid–liquid microextraction based on ionic liquid for spectrophotometric determination of titanium in environmental samples. J Ind Environ Chem. 2017;1(1):22-30
Abstract
A novel fast and simple dispersive liquid–liquid microextraction depended on ionic liquid (IL) is applied for the first time to preconcentrate trace amount of titanium as a prior step to its spectrophotometric determination. In this procedure, least volume of an IL (1-hexyl-3- methylimmidazolium bis(trifluormethylsulfonyl)imid) as the extractive solvent was dissolved in acetonitrile as the disperser solvent and the binary solution was then injected rapidly by a syringe into the water sample containing Ti(IV) complexed using 4-(2-benzothiazolyl-azo)2,2`- biphenyldiol (BTABPD) in the presence of sodium dodecyl sulphate (SDS) as the anti-sticking agent. The cloudy solution was formed and the Ti–BTABPD complex was extracted into the fine IL droplets. The droplets of extractant were settled at the bottom of a conical test tube after centrifuging, and the extracted phase was diluted with 100 μL of ethanol and measured spectrophotometrically at 626 nm. Parameters affect the formation of the complex and its extraction, as reagent concentration, pH, salt concentration, type and concentration of antisticking agent, and the type and volumes of extraction disperser solvents, which is optimized for the presented procedure. The enhancement factor of 250 was obtained for 25 mL of water under optimum conditions. The detection limit (LOD) of the procedure was found to be 0.06 ng mL−1 and the RSD (n=5) for 2.0 ng mL−1 of Ti(IV) was 1.53%.
Keywords
Ionic liquid, Dispersive liquid–liquid microextraction, Titanium determination, Spectrophotometry, Environmental and biological analysis
Introduction
The ninth most abundant element in earth’s crust is titanium, a natural constituent of rocks, sediments and soils [1]. The levels of titanium in rocks are less than 2.0% (w/w) [2]. The Ti concentrations found in river, estuarine and coastal waters [3] range from 0.005 to more than 4.8 mg L−1 and from 0.2 to 17 ng L−1 in ocean waters [1]. Minerals containing titanium are very resistant to chemical weathering in sedimentary and soil environments, allowing the common apply of titanium as a guide element to compare the mobility of the various elements [1].
In last decay, growing importance was observed for titanium presented in different industrial fields due to its chemical characteristics and particular physical. In solar energy cells, the main commercially available compound, titanium dioxide, is applied [4], as a photocatalyst in sterilization, water purification and air cleaning processes [5,6], as an ingredient of sunscreens, toothpastes, cosmetics, paints and plastics, and in the manufacture of building materials, missiles and aircrafts [7,8]. It also shows great important in the development of computer manufacture, antitumor agents [9], for drug delivery, and environmental cleanup [10]. Nanoparticles of titanium dioxide also represents durable photocatalytic activity, induced by UV light, causing photochemical degradation of organic compounds [6-11], indicating a potential apply in wastewater treatment plants [8].
In a recent work [12], depended on the existing quantitative toxicity data (e.g. LC50 or EC50) for the evaluation of the potential hazardous effects of nanoparticles [13], TiO2 was classified as “harmful”. This classification is depended on works that involved various groups of organisms (algae, bacteria, crustaceans, fish, nematodes and yeasts) and suggested that algae are the highly sensitive ones. The toxicity falls into the same classification, In the case of bulk TiO2, indicating a smallest LC50 value for algae, similar to the amount found for the nanoparticles formulation.
Despite good developments in the recent analytical equipments, direct determination of low levels in trace analytes is often a problem for analytical chemists and so, a sample-preparation step is needed. The continuous quest for novel sample preparation methods has led to the development of new methods, whose main advantages are their speed and negligible volume of solvents used. Reducing the volume of organic solvent as well as allowing sample extraction and preconcentration is the solvent microextraction technique effectively overcomes these difficulties to be done in a single step. In comparing with conventional procedures, solvent microextraction is simpler and faster, sensitive, and inexpensive, effective for the removal of interfering matrices effect. Solvent microextraction has been a form of solvent extraction with phase ratio values higher than 100 [14]. Compared with the conventional solvent extraction. Microextraction may provide poorer analyte recovery; instead the concentration in the organic phase greatly enriches [15].
As a big advantage, the volume of the applied organic solvent is highly reduced. In addition merely one step of operation is needed, therefore, loss of analytes vanish and problems of contamination.
A modified solvent microextraction procedure as dispersive liquid–liquid microextraction (DLLME) is applied and its acceptor-to-donor phase ratio is greatly reduced comparing with the other procedures [16-19]. In early DLLME procedures, the appropriate mixture of the disperser solvents and common organic extraction was injected firstly by syringe into aqueous samples having analytes. Thereby, a cloudy solution was formed and extraction occurred.
Classical liquid–liquid extraction (LLE) depended on ionic liquids (ILs) has been reported previously [20-28]. However, this procedure requires large amounts of IL, which is expensive. Single drop microextraction based on ILs was reported [29]. But in this method the sensitivity was analyte dependent because of its different partition coefficient and the relatively large viscosity of IL.
Different procedures were reported in the literature to determine titanium (IV) in trace levels [30-38]. Some of these procedures involve tedious methods and expensive instrumentation and are not feasible in a common laboratory. Various spectrophotometric procedures were reported in the literature for the analytical determination of titanium(IV). These procedures adopt relatively complicated and time sensitive or involve sensitive extraction steps prone to contamination at any stage of the analysis and hence require skilled analysts [9,39-53].
For the first time in DLLME, small volumes of a hydrophobic IL, namely, 1-hexyl-3-methylimmidazolium bis(trifluormethylsulfonyl)imid ([Hmim][Tf2N]), were applied as solvent extraction, which is dissolved in acetone as the disperser solvent and then dispersed into the sample solution having sodium dodecyl sulphate (SDS) as an anti-sticking agent to prevent sticking of IL to the test tube wall. Therefore, a cloudy solution of fine droplets of IL appeared.
To the best of our knowledge, there is no earlier literature presented on the performance of DLLME depended on IL is investigated to determine titanium(IV) in water samples applying spectrophotometric detection. The effect of different experimental parameters on the extraction was investigated. Further, in comparison with organic solvent extraction, it is much safer since only small volumes of IL are applied which is being considered as a ‘green solvent’ for several separation processes.
Experimental
Apparatus
A water bath with good temperature control and a centrifuge with 10 mL calibrated centrifuge tubes (Superior, Germany) were used to accelerate the phase separation process. An Orion research model 601 A/digital ionalyzer pH meter was used for checking the pH of solutions. Tabletop Low Speed Large Capacity Centrifuge model L-550 was used. A Perkin Elmer model 5300 DV; ICP-AES (Waltham, MA, USA) was used for all ICP-AES measurements. A Perkin-Elmer Lambda 12 UV/Vis spectrometer was used for recording absorbance spectra with 1.0 mm quartz cell.
Reagents and solutions
All chemical reagents were of analytical-reagent grade and deionized water was used for preparation of the sample solutions. A 3.14 × 10−3 M standard titanium(IV) solution was prepared by heating 0.06191 g of titanium dioxide with sulphuric acid and ammonium sulphate and finally diluted to 250 mL with double distilled water. Its final concentration was determined spectrophotometrically [54,55]. Working solutions were prepared daily by appropriate dilution of stock solution.
4-(2-benzothiazolylazo)2,2`-biphenyldiol (BTABPD) reagent was prepared applying conventional diazotization and coupling procedures [56]. A solution of 2.5 × 10−3 M of (BTABPD) was prepared by dissolving appropriate amounts of this reagent in ethanol.
A 1.0% (w/v) solution was prepared by dissolving the appropriate amount of SDS in doubly distilled water and a buffer solution of pH=3.9 was prepared by mixing 88 mL of acetic acid (0.1 M) and 13 mL of sodium acetate (0.1 M). Acetonitrile as a disperser solvent, [Hmim][Tf2N] as an extraction phase, SDS as the antisticking agent, acetic acid, sodium acetate, ethanol and sodium nitrate were purchased from Merck (Darmstadt, Germany).
DLLME procedure
A total of 500 μL of buffer pH=3.9, 250 μL of NaNO3 1.0 M and 250 μL of SDS 1.0% (w/v) were added into a 25 mL test tube with conical bottom containing titanium in the range of 5.0–90 ng mL−1. Then, 200 μL of BTABPD (2.5 × 10-3 M) as complexing agent was added to the solution and the 25 mL total volume was adjusted with doubly distilled water. The pinkish complex of titanium was formed. Then, 250 μL of binary solution containing 75 mg of [Hmim][Tf2N] (extraction solvent) and acetonitrile (disperser solvent) was injected rapidly into the sample solution using a syringe and a stable cloudy solution (water, acetonitrile and IL) was obtained. The Ti–BTABPD complex was extracted into the fine droplets of IL. The mixture was then centrifuged for 5.0 min at 4250 rpm. After this process fine droplets of [Hmim][Tf2N] were joined together and sedimented at the bottom of the conical test tube. After removing the whole aqueous solution, the extraction phase was diluted with 100 μL of ethanol (95%) and transferred to a 100 μL cell and the absorbance was measured at 626 nm.
Sample preparation
The certified reference materials analysed to determine the accuracy of the proposed procedure were in accordance with National Institute of Standard and Technology (NIST) and British Chemical Standard (BCS) guidelines. Sample of 1.0 g was transferred into 200 mL Borosil beaker, 25 mL of concentrated hydrochloric acid was added, followed by digestion on a sandbath for 1.0 h and evaporation to dryness. The residue was dissolved in 10–15 mL concentrated hydrochloric acid along with 0.5 g of ammonium persulphate. The solution was diluted with distilled water, filtered and finally diluted to the mark in a 100 mL standard flask with distilled water.
Plant sample
A known weight of the sample (i.e., 100 g) was ashed in a porcelain crucible at 450°C for 3 h and 10 g of ash was transferred into 200 mL Borosil beaker and digested in a sandbath with 100 mL concentrated hydrochloric acid and 20 mL concentrated nitric acid for about 1.0 h. The hot solution was centrifuged and the supernatant was decanted from any siliceous matter. Residue was boiled with 50 mL of 0.1 M hydrochloric acid and filtered, then the filtrate and washings were evaporated to dryness, and residue was taken up and diluted accurately to 100 mL with 0.1 M hydrochloric acid.
Waste water and sea water analysis
The effluent samples were filtered through the Whatman filter paper and were introduced into an extraction system.
Results and Discussion
Selection of IL
ILs are composed of asymmetrically substituted nitrogencontaining cations (e.g. imidazole, pyrrolidine, pyridine, . . . .) with inorganic anions (e.g. Cl−, BF4−, PF6−, (CF3SO2)2N−, . . . ). The range of available anion and cation combination could provide too various different ILs, so at first glance, perhaps it is challenging to select the desired IL but by considering the following experimental conditions, the selection is not very difficult: IL has to be water-immiscible for analyte extraction. ILs containing Cl−, BF4− and CF3SO3− are water miscible and ILs containing PF6−, (CF3SO2)2N− are water-immiscible. In addition, IL is a liquid in experimental conditions and have extraction capability of the interested compounds and higher density than water for LLE. ILs containing an imidazolium cation was chosen in the proposed work. ILs containing (CF3SO2)2N− and PF6− are hydrophobic and liquid in the experimental conditions. Generally, chosen IL has to be more immiscible in the sample solution to reduce extraction solvent consumption. Moreover, IL must produce sedimented phase at appropriate amounts. Since the sample volume was 10 mL so if [Hmim][Tf2N] was selected as IL, about 34 mg of it will be dissolved in the sample, while water solubility of [Hmim][PF6] is 75 mg/25 mL. Therefore, [Hmim][Tf2N] IL as the extraction solvent is chosen this study.
Effect of pH
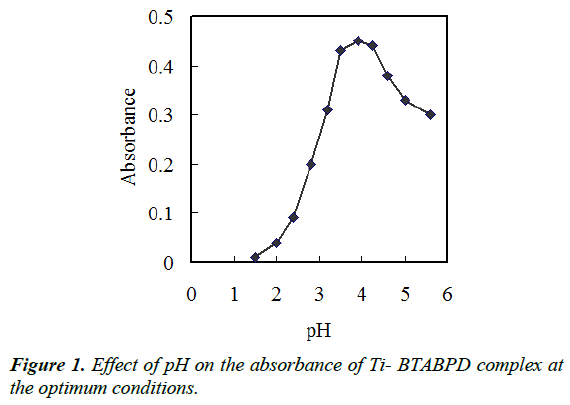
Separation of various metal ions by DLLME involves prior complex formation with sufficient hydrophobicity to be extracted into the small amounts of the IL phase, whereby the desired preconcentration is achieved. pH plays a distinctive role on metal-complex formation and subsequent extraction into IL phase. The pH effect on the titanium complex extraction from water samples was investigated in the range of 1.5–5.6. The results shown in Figure 1 reveal that the absorbance is initially increased by rising pH to 3.5 and then, absorbance starts to decrease after pH 4.25. Thus, pH 3.9 seems a proper choice for both complexation and extraction.
Effect of acetate/acetic acid buffer concentration
The buffer concentration as a function of absorbance was investigated in the range of 0.0–0.2 M. Absorbance initially increased up to 0.01 M of buffer and then approximately stayed constant till 0.15 M. A concentration of 0.10 M buffer was chosen for subsequent experiments.
Effect of reagent concentration
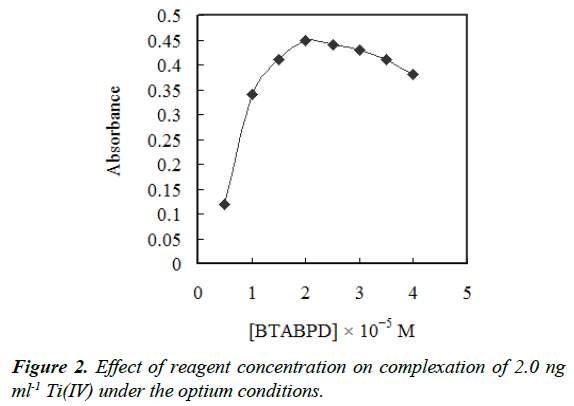
Also, the concentration effect of BTABPD was examined. The metal extraction efficiency as a function of the BTABPD concentration is represented in Figure 2. The results revealed that the extraction efficiency increased by increasing DMPAHPD concentration up to 2 × 10−5 M and remained nearly constant at higher concentrations. Therefore, this concentration was chosen as best.
Selection of anti-sticking agent and its concentration
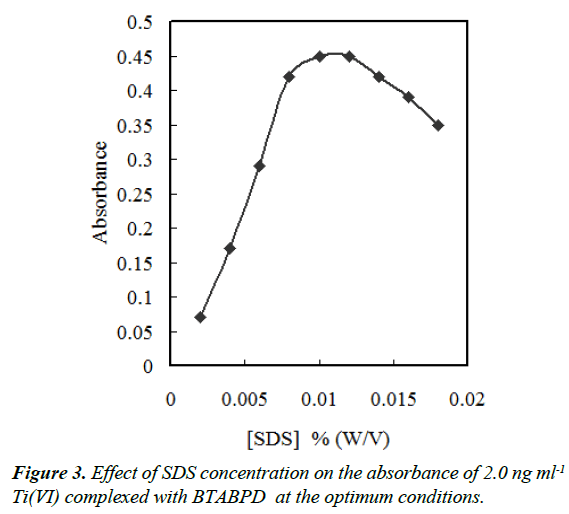
The sample solution containing fine particles of IL, some of the IL droplets have stuck on the wall of the test tube after centrifugation [21], so some extracted phase was lost. In order to overcome this problem, a surfactant was added into the sample solution. Therefore, molecules of surfactant surrounded fine particles of IL is formed during phase separation. Hence their interaction with the wall of the test tube decreased and consequently IL did not stick on it [21]. We have also investigated the effect of Triton X-114, Triton X-100, Tween -80, Tween 60 and SDS. The results indicated that all these surfactants were helpful and in the presence of them the stickiness decreased clearly. SDS was more effective than the others, perhaps due to its assisting onion-pair extraction of the titanium cationic complex and/or its solubilising effect on complexation (the precipitation elimination). In the presence of SDS, the absorbance initially increased till 0.012% (w/v) and then decreased due to dissolving of IL. According to Figure 3, a concentration of 0.01% (w/v) SDS was selected for subsequent experiments.
Effect of amount of the extraction solvent
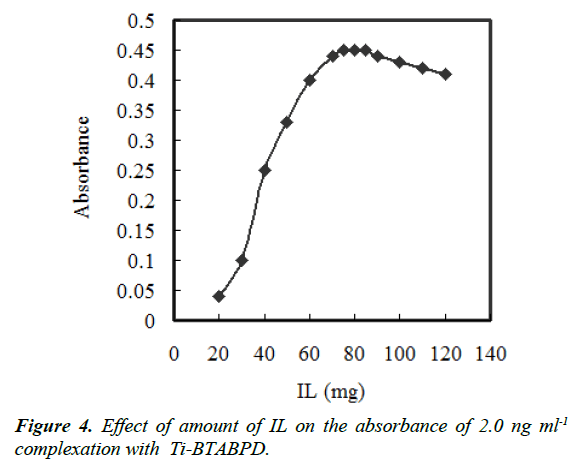
The effect of the extractant amount was examined. Solutions containing 500 μL of acetonitrile and different amounts of IL in the range of 10–120 mg were subjected to the same procedures. According to Figure 4 that indicates the curve of absorbance versus the amount of IL. Increasing IL concentration, the absorbance initially increases up to 70 mg, then after 85 mg it starts to decrease. This drop off is due to the rise of the sedimented phase volume. The high sensitivity was achieved using 75 mg of [Hmim][Tf2N] that was dissolved in 500 μL of acetonitrile disperser solvent. The amount of the sedimented phase was investigated using a microsyringe and was about 250 μL.
Effect of type and volume of the disperser solvent
The main criterion for the selection of disperser solvent is its miscibility in the extraction solvent and aqueous solution. In addition, the type of disperser directly affects the viscosity of the binary solvent. Thus, this solvent can control droplet producing and extraction efficiency. For investigating this effect, two various solvents as acetone and acetonitrile were tested. A series of sample solutions were investigated using 500 μL of each disperser solvent with 75 mg of the IL (extraction solvent). The obtained enhancement factors for these two dispersers indicate no statistical significant differences between them; however acetonitrile was chosen because it is more accessible than acetone.
The effect of the acetonitrile volume on the extraction recovery was also investigated. The different volumes of acetonitrile up to 800 μL with addition of 75 mg of [Hmim][Tf2N] were examined. At the first two tests, the droplets were big and the surface area was low. Therefore, the droplets rapidly settled at the bottom of the tube and low extraction efficiencies were achieved. As it is represented in Figure 4, the absorbance increases up to 100 μL of disperser solvent volume and after that it approximately stays constant. Thus, 250 μL of acetonitrile was selected as the proper amount.
Effect of salt concentration
For studying The influence of ionic strength on the performance of DLLME was studied. The NaNO3 concentration in the range of 0.0–0.3 M was investigated while other experimental conditions were kept constant. Increasing NaNO3 concentration, the extraction efficiency slowly increases due to the salting-out effect and then stays approximately constant. A concentration of 0.10 M NaNO3 was selected for subsequent experiments to increase the recovery.
Effect of centrifuge conditions
A series of solutions were examined at different rates of centrifugation. The rate of centrifugation was adjusted in the range of 1000–6000 rpm for 5.0 min. The absorbance slowly increases with increasing the rate up to 4000 rpm and after that, it approximately stays constant. Therefore, 4250 rpm was chosen as the best rate for centrifuging.
Absorbance was investigated at the optimum rate as a function of centrifugation time. Over 5.0 min, the absorbance was constant, showing complete transfer of IL phase to the bottom of the centrifuge tube. Therefore, 5.0 min were chosen as the best centrifugation time.
Selection of the diluting agent for IL phase
Diluting agent has to dissolve the IL and complex completely. We investigated the effect of acetone (40–90%) and ethanol (60–100%). When one of these diluting agents was added in different compositions, λmax of complex was slightly shifted due to changes of solvent polarity. So in each composition of diluent, the absorbance was measured at related λmax. In 60% acetone the maximum absorbance was obtained but IL phase could not be dissolved completely, so the solution was turbid, but in the presence of 95% ethanol the sample was clear and maximum absorbance was acquired. Therefore, ethanol 95% was chosen as a diluting agent.
Effect of diverse ions
The effect of different cations and the anions in the determination of titanium was investigated. Interference studies were carried out by measuring the absorbance of the extracted complex phase. The tolerance limit was set as the amount of foreign ion changing the absorbance by ± 5.0%. Titanium was extracted in the presence of associated alkali metals, alkaline earths, vanadium, molybdenum, niobium, tantalum, zirconium, iron, manganese, chromium and the large number of competitive ions at pH 3.9 and none of them has effected the absorbance of the titanium complex. This confirms that the KTi values are greater than KMn+ or KAn− for the competing metal cations or anions, which were determined independently at pH 3.9. The results, given in Table 1, show that the selectivity factor, KTi, (KTi = βK`e/ KMn+ or βK`e/ KAn−) for titanium complex had a high selectivity with most of the cations and anions. The data given in Table 1 show that moderate amounts of commonly occurring metal ions associated with titanium were tolerated.
Table 1.Tolerance ratio of diverse ions on thedetermination of 2.0 ng mL−1 of Ti(IV).
| Ion | Tolerance ratio |
|---|---|
| CH3COO−, benzoate | 14000 |
| NO3−,SO42−,Cl− | 10000 |
| PO43+, I−, Br− | 7500 |
| Na+ , K+ ,Ca2+ | 6000 |
| Cu2+, Co2+, Mn2+ | 5000 |
| Ni2+, Pb2+, Al3+ | 3750 |
| Ce3+, Zn2+ , Mg2+ | 2500 |
| Mg2+, Sr2+ Cd2+ | 1700 |
| As3+, Fe2+, Fe3+ | 1250 |
| MoO42−, WO42− | 750 |
| Cr3+↰, Ag↰+, Au3+↰ | 600 |
| Pd2+↰, Zr4+, Nb5+ | 450 |
| V5+, Sn2+, Y3+ | 300 |
Analytical characteristics
The analytical characteristics of the optimized procedure is summarized (Table 2) including regression equation, linear range, limit of detection (LOD), preconcentration and improvement factors. The RSD for six replicate measurements of 2.0 ng mL−1 of Ti(IV) was 1.53%, and for 3.0 ng mL−1 was 1.77%.
Table 2. Analytical features of the proposed method.
| Parameters | DLLME method | Before DLLME |
|---|---|---|
| Amount of acetonitrile (mL) | 0.5 | --- |
| pH | 3.9 | 3.9 |
| Optimum [BTAHQ] (M) | 2 × 10−5 | 5 × 10−4 |
| Reaction time (min) | 5 | 15 |
| Stirring time (min) | 5 | ---- |
| Beer’s range (ng mL−1) | 0.2 – 3.6 | 20000-250000 |
| Ringbom range (ng mL−1) | 0.5 – 3.3 | 30000-225000 |
| Molar absorptivity (L mol−1 cm−1) | 1.01 × 106 | 0.592 × 103 |
| Sandell sensitivity (ng cm−2) | 0.0044 | 0.76 |
| Regression equation a | ||
| Slope | 22.5 | 0.00132 |
| Intercept | -0.005 | 0.025 |
| Correlation coefficient (r) | 0.9995 | 0.9966 |
| RSD (%) | 1.53 | 2.25 |
| Detection limit, LOD (ng mL−1) | 0.06 | 5500 |
| Quantification limit, LOQ (ng mL−1) | 0.2 | 18000 |
| Preconcentration factor | 250 | |
| Improvement factor | 1706 |
Note: a: A = a+ bC, where C is the concentration of Ti(IV) in mg mL−1
The LOD, defined as CL =3SB/m where CL, SB, and m are the limit of detection, standard deviation of the blank and slope of the calibration graph, respectively, were 0.06 ng mL−1 for Ti(IV). Because the amount of Ti(IV) in 25 mL of sample solution is measured after preconcentration by DLLME in a final volume of 100 μL, the maximum preconcentration factor of the solution is 250. The molar absorptivity before DLLME was 0.592 × 103 L mol−1 cm−1 which increased greatly after DLLME due to preconcentration and improvement factors to 1.01 × 106 L mol−1 cm−1. The improvement factor, defined as the ratio of the slope of the calibration graph for the DLLME method to that of the calibration graph in aqueous media, was 1706 for Ti(IV).
A comparison of the proposed procedure with the previously reported ones for spectrophotometric determination of Ti(IV) (Table 3) [43-53] shows that the proposed procedure is faster, simpler, more sensitive and selective than the existing methods and that it provides a lower LOD and LOQ, a wider linear range, requires inexpensive instrumentation and consumables.
Table 3. A review of the spectrophotometric methods to indicate the advantages of the proposed method.
| Reagent | λmax [nm] | Remarks | ε, × 104 L mol−1 cm−1 | Ref. |
|---|---|---|---|---|
| Cetyltrimethylammonium, cetylpyridinium or tetradecyldimethylbenzylammonium cation | 420 | Involves an extraction step | (6-7) | [43] |
| Thiocyanate and cetyltrimethylammonium bromide | 421 | Involves an extraction step | 43019 | [44] |
| N-Phenyllaurohydroxamic acid and phenylflurone | 540 | Involves an extraction step | 2.33 | [45] |
| 2,6,7-Trihydroxylphenyl-fluorone derivatives,nitrilotriacetic acid and cetyltrimethylammonium bromide | 576 | Involves the formation of a quaternary complex | 19 | [46] |
| 2,4-Dihydroxybenzaldehyde isonicotinoyl hydrazone | 430-500 | Narrow Beer’s law range | 1.35 | [47] |
| 2,3-Dihydroxynaphthalene | 375 | Involves extraction and reextraction steps | 3.2 | [48] |
| N-Pivaloyl-p-chloro-phenylhydroxylamine | 380 | Involves an extraction step | 0.53 | [49] |
| 3-Hydroxy-2-methyl-1-(4-tolyl)-4-pyridone | 355 | Involves an extraction step;narrow Beer’s law range | 1.6 | [50] |
| N1-Hydroxy-N1, N2 -diphenylbenzamidine and thiocyanate | 400 | Involves an extraction step;narrow Beer’s law range | 2 | [51] |
| Chlorpromazine hydrochloride | 417 | Involves an extraction step;narrow Beer’s law range | 2.6 | [52] |
| Mixed-ligand titanium(IV)-fluoride-alizarin Comple | 513 | Involves an extraction step; narrow Beer’s law range |
7 | [53] |
| N'-(2-hydroxybenzylidene)-3-oxobutanehydrazide | 500 | Involves an extraction step;narrow Beer’s law range | 1.68 | [57] |
| BTAHQusingDLLME | 626 | Involves an extraction step | 100.1 | This work |
To evaluate the accuracy and precision of the proposed method, solutions containing three different concentrations of each of Ti(IV) were prepared. The assay procedure was analyzed in six replicates, and the relative standard deviation given as a percentage (RSD%) was obtained within the same day to evaluate the repeatability (intra-assay) and over five different days to evaluate the intermediate precision (inter-assay). The analytical results of the intra-day and inter-day precision and accuracy shows that the proposed procedure exhibit good repeatability and reproducibility.
Analysis of water samples
Concluded the optimization of all of the physical and chemical parameters, the proposed analytical procedure was applied to different types of water in order to assess its accuracy. No detectable levels of titanium in the tap, well, polluted, sea, river and mineral water samples were found. Each type of water was spiked with variable amounts of Ti(IV) to assess matrix effects. The results are recorded in Table 4. The relative recoveries of titanium from mentioned water samples at different spiking levels were between 98.2 and 102.2%. These results demonstrated matrices of these water samples, in our present context, had little effect on DLLME of titanium. The results obtained with the proposed method were in good agreement with those previously reported using ICP–AES, while Ti(IV) recoveries were highly satisfactory for all cases.
Table 4. Determination of titanium in the water samples by the proposed method.
| Sample | Ti(IV)Added ng mL−1 | Proposed method | ICP–AES method | ||
|---|---|---|---|---|---|
| Founda (ng mL−1) | Recovery (%) | Founda (ng mL−1) | Recovery (%) | ||
| Tap water | 0 | n.db | --- | n.db | |
| 0.2 | 0.203 ± 0.43 | 101.5 | 0.198 ± 0.59 | 99 | |
| 0.4 | 0.405 ± 0.65 | 101.25 | 0.396 ± 0.76 | 99 | |
| 0.8 | 0.791 ± 0.80 | 98.87 | 0.811 ± 0.89 | 101.38 | |
| Well water | 0 | n.db | --- | n.db | |
| 0.3 | 0.304 ± 0.44 | 101.33 | 0.305 ± 0.71 | 101.67 | |
| 0.6 | 0.609 ± 0.57 | 101.5 | 0.593 ± 0.80 | 99.83 | |
| 0.9 | 0.888 ± 0.78 | 98.67 | 0.909 ± 0.97 | 101 | |
| Polluted water | 0 | 0.06 | 0.059 | ||
| 0.25 | 0.304 ± 0.59 | 98.06 | 0.311 ± 0.39 | 100.81 | |
| 0.5 | 0.564 ± 0.71 | 100.71 | 0.562 ± 0.56 | 100.63 | |
| 0.75 | 0.805 ± 0.85 | 99.38 | 0.822 ± 0.68 | 101.66 | |
| Sea water | 0 | 0.088 | --- | 0.1 | --- |
| 0.15 | 0.160 ± 0.64 | 101.07 | 0.162 ± 0.47 | 101.25 | |
| 0.3 | 0.310 ± 0.55 | 100.39 | 0.317 ± 0.78 | 102.25 | |
| 0.45 | 0.464 ± 0.38 | 101.13 | 0.453 ± 0.91 | 98.47 | |
| River water | 0 | n.db | --- | n.db | --- |
| 0.175 | 0.173 ± 0.72 | 98.57 | 0.177 ± 0.79 | 101.14 | |
| 0.35 | 0.343 ± 0.54 | 97.85 | 0.348 ± 0.39 | 99.43 | |
| 0.55 | 0.558 ± 0.43 | 101.36 | 54.85 ± 0.54 | 99.73 | |
| mineral water | 0 | n.db | ---- | n.db | ---- |
| 0.275 | 0.273 ± 0.36 | 99.27 | 0.276 ± 0.67 | 100.37 | |
| 0.55 | 0.547 ± 0.63 | 99.45 | 0.546 ± 0.92 | 99.27 | |
| 0.825 | 0.829 ± 0.47 | 100.48 | 0.842 ± 0.59 | 99.06 | |
Note: a: Mean ± SD (n = 5). b: Not detected. Results average of six consecutive measurements. Paired t-test found value=1.36; table value=2.56 for 95% confidence level
Analysis of the standard and environmental samples
To test the accuracy and applicability of the proposed procedure for the analysis of real samples, some reference materials of steel, and soil, plant samples from the industrial area of Shoubra, Egypt were analysed. Matrix interference was verified by comparison of the slopes of calibration graphs with those using standard addition method. The results of the analysis are recorded in Table 5. The performance of the proposed procedure was assessed by calculation of the t- value (for accuracy) and F- test (for precision) compared with ICP–AES procedure. The mean values were obtained in a Student’s t- and F- tests at 95% confidence limits for five degrees of freedom [57,58]. The results indicated that the calculated values (Table 5) did not exceed the theoretical values. A wider range of determination, higher accuracy, more stability and less time consuming, indicates the advantage of the proposed procedure over other ones.
Table 5. Determination of titanium in standard samples.| Sample | Certified value (%) | Founda (%) DLLME | ICP-AES | t- testb | F-value |
| Titanium | 1 | 1.021 ± 0.05 | 1.032 ± 0.03 | 1.62 | |
| Titanium | 1.8 | 1.783 ± 0.03 | 1.776 ± 0.05 | 3.24 | |
| BCS 321 mild steel | 0.12 | 0.116 ± 0.03 | 0.123 ± 0.04 | 1.48 | |
| BCS 324 mild steel | 0.03 | 0.031 ± 0.01 | 0.031 ± 0.06 | 2.98 | |
| NBS 77A burnt refractory | 2.93 | 2.944 ± 0.04 | 0.292 ± 0.04 | 1.79 | |
| Silicon aluminium alloy | 0.18 | 0.185 ± 0.02 | 0.182 ± 0.03 | 3.74 | |
| Environmental samples (Ti in µg mL−1) | |||||
| Soil sample (mg) (Benha, Egypt) | 7.523 ± 0.05 | 7.505 ± 0.02 | 1.55 | ||
| Plants | 26.84 ± 0.05 | 26.62 ± 0.07 | 3.08 | ||
| Pulp and paper | 63.33 ± 0.05 | 63.65 ± 0.07 | 1.82 | ||
| Paint and pigment | 190.8 ± 0.05 | 190.9 ± 0.08 | 3.74 |
Note: a: Mean ± SD (n = 6).b: Tabulated t-value for five degrees of freedom at P (0.95) is 2.57; c: Tabulated F-value at P (0.95) is 5.05
Conclusion
For the first time, the use of DLLME based on IL for preconcentration of titanium from real water samples is proposed as a prior step to their determination by spectrophotometric procedure. The proposed procedure is simple, rapid, sensitive, low cost, low LOD and has low toxicity since only very small volumes of an IL as a ‘green extraction solvent’ is used as a replacement of environmentally damaging organic solvents. Also the use of spectrophotometry as a detection system has a low operational cost in comparison with other procedure as FAAS, ICP/OES, and ICP-AES.
References
- Skrabal SA, Terry CM. Distributions of dissolved titanium in porewaters of estuarine and coastal marine sediments. Mar Chem. 2002;77(2-3):109–22.
- Lovern SB, Stricker JR, Klaper R. Behavioral and physiological changes in Daphnia magna when exposed to nanoparticle suspensions (Titanium Dioxide, Nano-C60, and C60HxC70Hx). Environ Sci Technol. 2007;41(12):4465–70.
- Skrabal SA. Distributions of dissolved titanium in Chesapeake Bay and the Amazon river estuary. Cosmochim Acta. 1995;59(12):2449–58.
- Shipway AN, Katz E, Willner I. Nanoparticle arrays on surfaces for electronic, optical and sensor applications. Chem Phys Chem. 2000;1(1):18–52.
- Lovern SB, Klaperm R. Daphnia magna mortality when exposed to titanium dioxide and fullerene (C60) nanoparticles. Environ Toxicol Chem. 2006;25:1132–7.
- Rinke KH, Simon M. Ecotoxic effect of photocatalytic active nanoparticles (TiO2) on algae and daphnids. Sci Pollut Res. 2006;13(4):225–32.
- Federici G, Shaw BJ, Handy RD. Toxicity of titanium dioxide nanoparticles to rainbow trout, (Oncorhynchus mykiss): Gill injury, oxidative stress, and other physiological effects. Aquat Toxicol. 2007;84(4):415–30.
- Kika FS, Themelis DG. Selective stopped-flow sequential injection method for the spectrophotometric determination of titanium in dental implant and natural Moroccan phosphate rock. Talanta. 2007;71(3):1405–10.
- Cai R, Kubota Y, Shuin T, et al. Introduction of cytotoxicity by photoexcited TiO2 particles. Cancer Res. 1992;52(8):2346–8.
- Masciongioli T, Zhang WX. Environmental technologies at nanoscale. Environ Sci Technol. 2003;37(5):102A–8A.
- Caruso RA, Antonietti M, Giersig M, et al. Modification of TiO2 network structures using a polymer gel coating technique. Chem Mater. 2001;13(3):1114–23.
- Kahru A, Dubourguier HC. From ecotoxicology to nanoecotoxicology. Toxicology. 2010;269(2-3):105–19.
- European Commission. Technical Guidance Document in Support of Commission Directive 93/67/EEC on Risk Assessment for New Notified Substances. Part II. Environmental Risk Assessment, Office for Official Publications of the European Communities, Luxembourg. 2003.
- Carasek E, Tonjes JW, Scharf M. A new method of microvolume back-extraction procedure for enrichment of Pb and Cd and determination by flame atomic absorption spectrometry. Talanta. 2002;56(1):185–9.
- Jahromi EZ, Bidari A, Assadi Y, et al. Dispersive liquid-liquid microextraction combined with graphite furnace atomic absorption spectrometry: Ultra-trace determination of cadmium in water samples. Anal Chim Acta. 2007;585(2): 305–11.
- Rezaee M, Assadi Y, Hosseini MRM, et al. Determination of organic compounds in water using dispersive liquid-liquid microextraction. J Chromatogr A. 2006;1116(1-2): 1–9.
- Berijani S, Assadi Y, Anbia M, et al. Dispersive liquid-liquid microextraction combined with gas chromatography-flame photometric detection. Very simple, rapid and sensitive method for the determination of organophosphorus pesticides in water. J Chromatogr A. 2006;1123(1):1–9.
- Kozani RR, Assadi Y, Shemirani F, et al. Part-per-trillion determination of chlorobenzenes in water using dispersive liquid-liquid microextraction combined gas chromatography-electron capture detection. Talanta. 2007;72(2):387–92.
- Gharehbaghi M, Shemirani F, Baghdadi M. Dispersive liquid–liquid microextraction and spectrophotometric determination of cobalt in water samples. Inter J Environ Anal Chem. 2008;88:513–23.
- Visser AE, Swatloski RP, Reichert WM, et al. Traditional extractants in nontraditional solvents: groups 1 and 2 extraction by crown ethers in room-temperature ionic liquids. Ind Eng Chem Res. 2001;39(10):3596–604.
- Visser AE, Swatloski RP, Griffin ST, et al. Liquid/liquid extraction of metal ions in room temperature ionic liquids. Sep Sci Technol. 2001;36(5-6):785–804.
- Visser AE, Swatloski RP, Reichert WM, et al. Task-specific ionic liquids incorporating novel cations for the coordination and extraction of Hg2+ and Cd2+: synthesis, characterization, and extraction studies. Environ Sci Technol. 2002;36(11):2523–9.
- Li ZJ, Wei Q, Yuan R, et al. A new room temperature ionic liquid 1-butyl-3-trimethylsilylimidazolium hexafluoro-phosphate as a solvent for extraction and preconcentration of mercury with determination by cold vapor atomic absorption spectrometry. Talanta. 2007;71(1): 68–72.
- Chun S, Dzyuba SV, Bartsch RA. Influence of structural variation in room-temperature ionic liquids on the selectivity and efficiency of competitive alkali metal salt extraction by a crown ether. Anal Chem. 2001;73(15):3737–41.
- Wei GT, Yang Z, Chen CJ. Room temperature ionic liquid as a novel medium for liquid/liquid extraction of metal ions. Anal Chim Acta. 2003;488:183–92.
- Luo H, Dai S, Bonnesen PV. Solvent extraction of Sr2+ and Cs+ based on room-temperature ionic liquids containing monoaza-substituted crown ethers. Anal Chem. 2004;76(10):2773–9.
- Germani R, Mancini MV, Savelli G, et al. Mercury extraction by ionic liquids: temperature and alkyl chain length effect. Tetrahedron Lett. 2007;48:1767–69.
- Papaiconomou N, Lee JM, Salminen J, et al. Selective extraction of copper, mercury, silver and palladium ions from water using hydrophobic ionic liquids. Ind Eng Chem Res. 2008;47(15):5080–4.
- Liu JF, Chi YG, Jiang GB, et al. Ionic liquid-based liquid-phase microextraction, a new sample enrichment procedure for liquid chromatography. J Chromatogr A. 2004;1026:143–7.
- Vukomanović DV, Gary WV. New methods for trace titanium determination by adsorptive preconcentration voltammetry with pyrocatechol violet. Fresenius'. J Anal Chem. 1994;350(6):352–8.
- Gawry M, Golimowski J. Sensitive and very selective determination of titanium by adsorptive-catalytic stripping voltammetry with methylthymol blue, xylenol orange and calcein. Anal Chim Acta. 2001;427(1):55–61.
- Einhäuser TJ, Pieper TG, Keppler BK. Titanium determination in human blood plasma by ICP-OES, longitudinally, and transversally heated Zeeman ETAAS. J Anal At Spect. 1998;13(10):1173–6.
- Abbasi SA. Titanium as pollutant and a new method for its spectrophotometric and atomic absorption spectrometric microdetermination with N-p-methoxyphenyl-2-furylacrylohydroxamic acid. Anal Lett. 1987;20(11):1697–1717.
- Agrawal YK, Sudhakar S. Extractive spectrophotometric and inductively coupled plasma atomic emission spectrophotometric determination of titanium by using dibenzo-18-crown-6. Talanta. 2002;57(1):97–104.
- Andrade JBD, Nunes GS, Veiga MP, et al. Spectrophotometric and inductively coupled plasma atomic emission spectrometric determination of titanium in ilmenites after rapid dissolution with phosphoric acid. Talanta. 1997;44(2):165–8.
- Garcia IL, Jerez IA, Campillo N, et al. Determination of tin and titanium in soils, sediments and sludges using electrothermal atomic absorption spectrometry with slurry sample introduction. Talanta. 2004;62(2):413–9.
- Nagaosa Y, Segawam SI. Reversed phase HPLC determination of titanium(IV) and iron(III) with sodium 1,2-dihydroxybenzene-3,5-disulfonic acid. J High Res Chromatogr. 1994;17(17):770–2.
- Bagur G, Sanchez-Vinas M, Gazquez D. Determination of titanium(lV) as an additive in organic matrices by reversed-phase high-performance liquid chromatography with 5,5′-methylenedisalicylohydroxamic acid. J Chromatogr Sci. 1997;35(3):131–4.
- Cresser MS. Solvent extraction in flame spectroscopic analysis. London: Butterworths; 1978.
- Purohit R, Devi S. Spectrophotometric determination of titanium(IV) using chromotropic acid and a flow injection manifold. Analyst. 1992;117(7):1175–7.
- Xiong Y, Zhou ZR, Wu FH. Kinetic spectrophotometric determination of trace titanium(IV) based on oxidation discoloration of acid chrome blue K with hydrogen peroxide. J Chin Univ Mining & Tech. 2007;17:418–23
- Zhai QZ, Sun FH. Determination of trace titanium with titanium(IV)-(DBC-arsenazo)-potassium bromate system by catalytic-kinetic spectrophotometry. J Anal Chem. 2008;63(11):1057–60.
- Vojković V, Zivcić VA, Drusković V. Spectrophotometric determination of titanium(IV) by extraction of its thiocyanate complex with cationic surfactants. Spect Lett. 2004;37(4):401–20.
- Tarafder PK, Thakur R. Micelle mediated extraction of titanium and its ultra-trace determination in silicate rocks. Talanta. 2008;75(1):326–31.
- Gunawardhana HD. Extraction and spectrophotometric determination of titanium(IV) with N-phenyllaurohydroxamic acid and phenylflurone. Analyst. 1983;108(1289):952–8.
- Wang DJ, Zhuang JY, Xie ZH, et al. Spectrophotometric study on the quaternary complex of titanium(IV) with secondary ligands, 2,6,7-trihydroxylphenyl-fluorone derivatives and cetyltri-methylammonium bromide. Microchim Acta. 1992;108(1-2): 79–91.
- Babaiah O, Rao CK, Reddy TS, et al. Rapid, selective, direct and derivative spectrophotometric determination of titanium with 2,4-dihydroxybenz-aldehyde isonicotinoyl hydrazone. Talanta. 1996;43(4)551–8.
- Mondal RK, Tarafder PK. Extractive spectrophotometric determination of titanium in silicate rocks, soils and columbite–tantalite minerals. Microchim Acta. 2004;148(3-4):327–33.
- Baccan N. Extraction and spectrophotometric determination of titanium(IV) with N-pivaloyl-p-Cl-phenylhydroxylamine in chloroform. Fresenius Zeitschrift fur Anal Chem. 1983;316:796–9.
- Tamhina B, Vojković V. Extraction and spectrophotometric determination of titanium(IV) with 3-hydroxy-2-methyl-1-(4-tolyl)-4-pyridone. Microchim Acta. 1986;88(1-2):135–45.
- Yigzaw Y, Chandravanshi BS. Extraction and spectrophotometric determination of titanium(IV) with N1-hydroxy-N1,N2 -diphenylbenzamidine and thiocyanate. Microchim Acta. 1996;124(1-2):81–7.
- Tarasiewicz HP, Tarasiewicz M, Misiuk W. Spectrophotometric determination of titanium(IV) with chlorpromazine hydrochloride. Microchem J. 1984;29(3): 341–4.
- Nunez RL, Mochon MC, Perez AG. Extraction and spectrophotometric determination of titanium(IV) with alizarin and fluoride. Talanta. 1986;33(7):587–91.
- Vogle F, Weber E. Host guest complex chemistry–macrocycles, synthesis, structure, applications. Berlin: Springer; 1985
- Agrawal YK, John KT. Extraction and spectrophotometric determination of titanium(IV). Analyst. 1985;110(1):57–9.
- Amin AS. The surfactant-sensitized analytical reaction of niobium with some thiazolylazo compounds. Microchem J. 2000;65(3):261–7.
- Srilalitha V, Prasad ARG, Kumar KR, et al. A new spectrophotometric method for the determination of trace amounts of titanium(IV). Chem Tech. 2010;8:15–24.
- Miller JN, Miller JC. Statistics and chemometrics for analytical chemistry. England: Prentice Hall; 2005.