Research Article - Biomedical Research (2017) Volume 28, Issue 3
Ultrasound-guided versus computed tomography-controlled transcranial drilling and catheter drainage on collagenase-induced intracerebral haemorrhage in rats
Li Chen1#, Xuan Zhou1#, Cong Feng1#, Xiang Cui2, Lili Wang1 and Tanshi Li1*1Department of Emergency, Chinese PLA General Hospital, Beijing, PR China
2Department of Orthopaedics, Chinese PLA General Hospital, Beijing, PR China
#These authors contributed equally to this work
- *Corresponding Author:
- Tanshi Li
Emergency Department
Chinese PLA General Hospital
Beijing, PR China
Accepted date: August 25, 2016
Abstract
Purpose: We evaluated accuracy and effect of ultrasound (US)-guided transcranial drilling and catheter drainage versus computed tomography (CT)-controlled interventions in the collagenase-induced intracerebral haemorrhage in rats.
Method: 120 male Sprague Dawley (SD) rats were randomly divided into four groups: group A, group N, group B and group C. Group A, group B and group N underwent collagenase-induced Intracerebral Haemorrhage (ICH) surgery procedure as animal model groups. Group C received a 10 μl saline injection with the same surgical procedure as the control group. US-guided transcranial drilling and catheter drainage were performed on group A. CT-controlled transcranial drilling and catheter drainage were performed on group B. Then group A, group B and group N were evaluated by ultrasound and CT. All groups were observed and assessed the behavior tests and mortality for 4 weeks. The remains of all groups were put to death after assessment. Apoptosis of neuron cells were identified by hematoxylin and eosin stain and immunohistochemistry.
Results: The mortality of each group showed no significant difference (p>0.05). Behavioral testing showed that there was no significant difference between group A and B (p>0.05). The expression of NFkB and caspase-3 revealed that there was a few apoptosis of neuron cells both in group A and B.
Conclusions: US-guided transcranial drilling and catheter drainage are as accurate as CT-controlled transcranial drilling and catheter drainage. It could result in a significant reduction of procedure expenditure under the avoidance of radiation and show the same therapeutic effect.
Keywords
Transcranial drilling and catheter drainage, Ultrasound, Computed tomography, Behavioral test, Collagenase-induced Intracerebral hemorrhage.
Introduction
Intracerebral hemorrhage (ICH) is a common and potentially disabling event worldwide. There is an approximate 50% mortality rate during the month following ICH [1]. High morbidity after ICH would impact the patients’ quality life for a long time. The understanding of cerebrovascular events during a brain hemorrhage is particularly important for the effective medications and treatments [2]. Computed tomography (CT) and magnetic resonance imaging (MRI) are well-established techniques that are used in clinical imaging investigations of hematoma area and development [3-5]. However, the techniques are too large to carry. Considering ultrasound is the most widely used technique for detecting hematoma in the early stage [6], our current study aimed to use ultrasound technique for the investigation of cerebrovascular events during a brain hemorrhage in a collagenase-induced ICH in rats. We want to find out one quicker and accurate treatment for early ICH, and simpler and more feasible examination for delay ICH.
Materials and Methods
Animal model
Animals were housed and treated in accordance with Chinese national guidelines for the care of laboratory animals and was approved by the Animal Care and Use Committee of the Chinese Academy of Military Medical Science.
A total of 120 young male Sprague Dawley (SD) rats (Laboratory Animal Center, Academy of Military Medical Sciences, Beijing, China) were used in this study. Animals weighing 250-280 g, aged 4-7 months, were housed individually with a 12 h light/dark cycle and given access to food and water ad libitum in a temperature-controlled environment (20-22°C), randomly divided into four groups: group A, group N, group B and group C. The performance of all groups was listed as follows.
Collagenase-induced ICH surgery procedure
Group A, group B and group N underwent collagenase-induced ICH surgery procedure as animal model groups. The protocol of collagenase-induced ICH was modified based on an established method [7]. Briefly, animals were anesthetized with 10% chloral hydrate (0.4 mg/kg for induction and 0.02 mg/kg for maintenance) and placed into a head-holder. Maintained the body temperature at 37°C by a heating lamp to prevent hypothermia during the surgery. Blood pressure, electrocardiography (ECG), blood pH PCO2 and PO2 were monitored and maintained within normal physiological limits. Group A, group B and group N were infiltrated with 1% lidocaine at the scalp area, explored a midline scalp incision. A burr hole was drilled through the skull. The injection sites were 3 mm right to midline, 1 mm posterior to the anterior fontanelle, and 5 mm below the surface of the skull. Collagenase (IV; Sigma, America) dissolved in saline was infused over 2 min. After infusion, the hole was sealed with bone wax. The wound was sutured, and the animal was then transferred to machine lab for the first scanning after surgery. After evaluated of bleeding site and severity, group A underwent US-guided transcranial drilling and catheter drainage therapy. Group B underwent CT-controlled transcranial drilling and catheter drainage. Group A, group B and group N were evaluated the bleeding part and severity by ultrasound and CT. Group C received a 10 μl saline injection with the same surgical procedure as the control group.
Behavioral tests
Behavioral tests were conducted at 1 week, 2 weeks, 3 weeks and 4 weeks after ICH surgery procedure.
The Bederson score was calculated as follows: Rat tail was lightly grasped 10 cm above the desktop, and the score was graded as the following: 0, no neurological deficit; 1, flexion of the contralateral wrist and elbow and flexion adduction of the contralateral shoulder; 2, signs in score 1 plus reduced resistances when pushing toward the paralyzed side; 3, circling toward the paralyzed side when acting (tail-chasing like) [8].
The Longa score was graded as the following: 0, no neurological deficit; 1, left front paw cannot fully extend; 2, walking in circle toward the contralateral side; 3, dumping toward the contralateral (paralyzed) side when walking; and 4, cannot autonomously walk with certain consciousness loss [9].
The beam-walking score was graded as the following: rats were placed 10 cm above the ground on an 80 × 2.5 × 2.5 cm stick to be allowed to walk, and the scores were graded as follows: 0, jump onto the balance beam and walk without falling; 1, can jump onto the balance beam and walk with less than 50% chances of falling; 2, can jump onto the balance beam and walk with more than 50% chance of falling; 3, can jump onto the balance beam with help from the ipsilateral hind limbs but cannot move forward due to the paralyzed contralateral hind limbs; 4, cannot walk on the balance beam but can sit on it; and 5, fall after being placed on the balance beam [10].
All groups were observed the mortality for 4 weeks. The remains of all groups were put to death at the 4 weeks after ICU.
Hematoxylin and eosin staining (HE staining)
At 4 weeks after ICU, after deeply anesthetized and perfused with 4% paraformaldehyde, the remains were placed in supine position. Blood and cerebrospinal fluid samples were draw and stored in a cryostat at -80°C for the later surgery. The skull was opened, and the whole brain was dissected. After removing the cerebellum, the brain sample was placed into the same fixative solution and fixed for another 24 h, embedded in paraffin, and finally cut into 4 μm thickness serial sections. Hematoxylin and eosin stain in accordance with the general steps to complete.
Immunohistochemistry
To evaluate the apoptosis of brain tissue, the brain sections were incubated with 5% bovine serum albumin (BSA, Sigma, USA) to block nonspecific binding and incubated with NF-kB antibody and caspase-3 antibody (Bioworld Consulting Laboratories, USA). The sections were incubated with biotinylated anti-mouse IgG (NF-kB, 1:200, Vector Laboratories, USA; caspase-3, 1:400, Chemicon International, USA) for 1 h at 37°C, followed by incubation with avidinbiotinperoxidase complex (ABC, 1:100, Vector Laboratories, USA) for 1 h at 37°C. Immunoreactivity was visualized with diaminobenzene (DAB, Boster Biotech, China).
Reverse transcription PCR (RT-PCR)
Brain tissues of each group preserved in -80°C were detected by RT-PCR. The extraction of total RNA and the process of Reverse transcription PCR were in strict accordance with the TaKaRa instruction. Primer orders were designed by Sangon Biotech (Shanghai) Co. NF-kB upstream: 5’- CGAGAFFAFCACAGATACCAC-3’, downstream: 5’- CGCTTCTTCACACACTGGATT-3’, amplification product 228 bp. Caspase-3 upstream: 5’- GGCATGGAGAACACTGAAAAC-3’, downstream: 5’- GCGAATCTGTTTCTTTGCATG-3’, amplification 786 bp. β- actin upstream: 5’-TCTACAATGAGCTGCGTGTGG-3’, downstream: 5’-GGAACCGCTCATTGCCAATG-3’, amplification product 499 bp.
The amplification process of NF-kB: predenaturation 5 min at 94°C, then 30 cycles of denaturation 30 s at 94°C annealing 30 s at 60°C, and elongation 40 s at 72°C, lastly with elongation 10 min at 72°C. The amplification process of bactin: predenaturation 5 min at 94°C, then 30°C cycles of denaturation 30 s at 94°C, annealing 30 s at 55°C, and elongation 40 s at 72°C, lastly with elongation 10 min at 72°C. The results were referred to β-actin, electrophoresed in 2% agarose-gel, analyzed by gel image machine, then quantified and saved by photograph. The index number of mRNA (RI)=the mRNA scanning value/β-actin mRNA scanning value.
Statistical analysis
Data were expressed as mean ± standard deviation (Mean ± SD). All data analysis was performed by SPSS 20.0. ANOVA was used for multiple-group comparison with post hoc Tukey test. P<0.05 was considered statistically significant.
Image analysis
Under the microscope (10 × 40), NF-kB +/caspase-3+ nuclei close to the hematoma were captured in 5 randomly selected sections from each animal by Olympus Microscope IX73, and analyzed by Image Analysis Software (Image J 1.29X).
Results
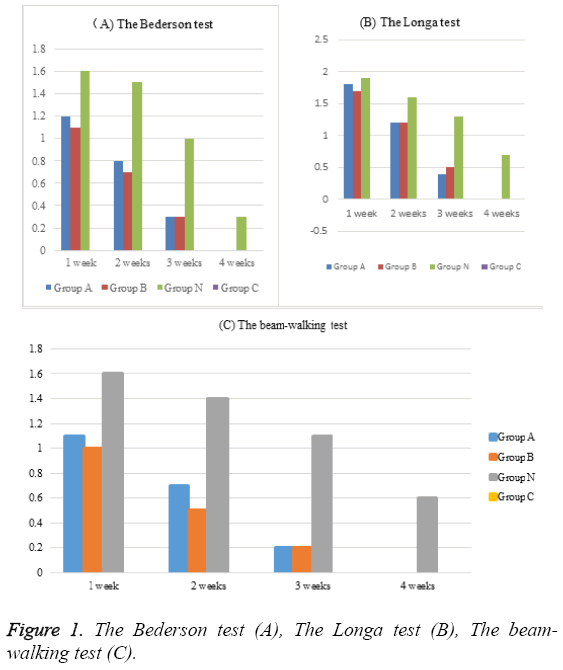
There are 120 rats before the study and 8 were dead after the ICH procedure (2 in group A at 1 day after surgery, 2 in group B at 1 day after surgery, 4 in group N within 3 days after ICH procedure). The survival rate of group A was 93.33%, that of group B was 93.33%, that of group N was 90%, that of group C was 100%. The behavioral tests of all groups were listed in Table 1 and Figure 1. Group A, B and N as collagenaseinduced ICH group exhibited a significant higher scores in the Bederson test, the Longa test and the beam-walking test compared to group C as control group (p<0.05). The behavioral scores of group A exhibited no significant difference in the Bederson test, the Longa test and the beamwalking test compared to group B (p>0.05). The behavioral scores of group A and group B exhibited significant lower scores in the Bederson test, the Longa test and the beamwalking test compared to group N (p<0.05).
| Tests | Group | 1 week | 2 weeks | 3 weeks | 4 weeks |
|---|---|---|---|---|---|
| The Bederson test | Group A | 1.2 ± 0.45 | 0.8 ± 0.67 | 0.3 ± 0.25 | 0.0 ± 0.00 |
| Group B | 1.1 ± 0.45 | 0.7 ± 0.32 | 0.3 ± 0.21 | 0.0 ± 0.00 | |
| Group N | 1.6 ± 0.78 | 1.5 ± 0.82 | 1.0 ± 0.23 | 0.3 ± 0.55 | |
| Group C | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | |
| The Longa test | Group A | 1.8 ± 0.67 | 1.2 ± 0.92 | 0.4 ± 0.31 | 0.0 ± 0.00 |
| Group B | 1.7 ± 0.72 | 1.2 ± 0.65 | 0.5 ± 0.62 | 0.0 ± 0.00 | |
| Group N | 1.9 ± 0.78 | 1.6 ± 0.66 | 1.3 ± 0.54 | 0.7 ± 0.78 | |
| Group C | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | |
| The beam-walking test | Group A | 1.1 ± 0.36 | 0.7 ± 0.82 | 0.2 ± 0.65 | 0.0 ± 0.00 |
| Group B | 1.0 ± 0.34 | 0.5 ± 0.52 | 0.2 ± 0.25 | 0.0 ± 0.00 | |
| Group N | 1.6 ± 0.67 | 1.4 ± 0.48 | 1.1 ± 0.24 | 0.6 ± 0.82 | |
| Group C | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 |
Table 1: The behavioral tests of all groups.
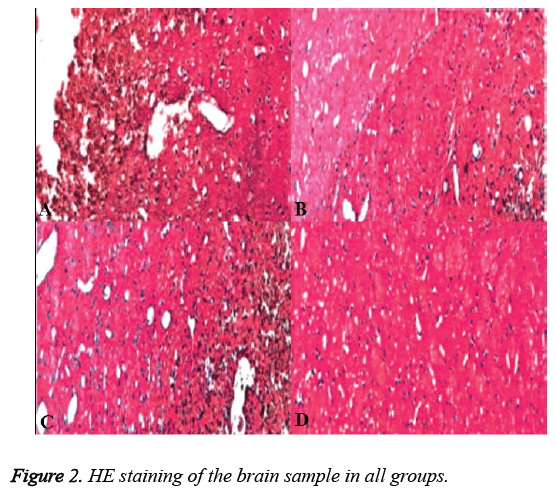
HE staining revealed that neuron cells near the needle track in group N were replaced by several sizes of cavities, glial cells and collagen fibers around the cavities formed glial scar (Figure 2A). HE staining revealed that a few neuron cells near the needle track in group A and B were hypertrophy and the branch were reduced (Figure 2B and Figure 2C). While in group C the neuron cells arranged in order and norm morph (Figure 2D). Immunohistochemistry revealed that NF-kB and Caspase-3 showed the most positive expression in group N, while that showed the numbers were reduced in group A and B. There was no NF-kB or Caspase-3 expressed in group C.
The mRNA expression levels of NF-kB and Caspase-3 were similar with the result of immunohistochemistry. The mRNA expression levels of NF-kB and Caspase-3 in group A were similar with that in group B, while were significantly higher than that in group C, lower than that in group N (Table 2).
| Group A | Group B | Group N | Group C | |
|---|---|---|---|---|
| NF-kB | 0.45 ± 0.23 | 0.43 ± 0.31 | 1.07 ± 0.17 | 0.02 ± 0.01 |
| Caspase-3 | 0.46 ± 0.18 | 0.47 ± 0.21 | 1.04 ± 0.08 | 0.01 ± 0.01 |
Table 2: The association between NF-kB and Caspase-3 of all groups.
Discussion
Our study consisted on histological characterization and behavioral testing of our ICH model associated with the therapy of ICH up to 2 months. The results extended our knowledge of neuron cells changes during haemorrhage and pioneered a new technique in collagenase-induced ICH animal model [11,12]. ICH is the most commonly produced in rat by either infusing autologous blood or collagenase [13]. In this study, we chose collagenase IV to induce ICH for the obviously neurological deficit and a high persistence [14]. In our study, neurological deficit caused by hematoma were observed in group A, B and N, especially in group N. It could see that the behaviour tests of group N had higher scores and gentler downward trend compared to group A and B. Combined with results of HE staining and immunohistochemistry, it showed that ICH would cause the irreversible damage of brain tissue and neuronal apoptosis. The faster appropriate therapy is given, the better recover we get [15]. The mortality of group N was the highest in the animal model groups, as there was no any therapy for group N after ICH procedure. We compared the effect of US-guided and CTcontrolled transcranial drilling and catheter drainage on collagenase-induced ICH in rats, the mortality showed that there was no significant difference between group A and B. Ultrasound could be the new diagnostic and therapeutic technique and extend in the clinical application for ICH [16].
For further studying the brain damage of each group, we analysed the apoptosis of neuronal cells in each group. Many reports have proved that haemorrhage would induce delayed brain injury even when the haemorrhage was removed [17]. The apoptosis of neuronal cells was associated with the bleeding and persistence time of haemorrhage [18]. Previous reports suggested that the expression of NF-kB and caspase-3 were associated with the injury of granule cell [19,20]. NF-kB exists in cerebrovascular endothelial cells, neuronal cells and glial cells and would be activated by ischemia of the central nervous system [21]. It hypothesized that NF-kB involved in regulating gene transcription function, mediated Fas associated death domain protein (FADD), activated Caspase-3 and finally induced apoptosis [22,23]. Caspase-3 (named cysteine protease 32) which is one of the most important caspase, also belongs to interleukin-1-β convertase (ICE) family [24], is relevant to apoptosis of neuronal cells after intracerebral haemorrhage [25,26]. Caspase-3 plays an important role in protein hydrolysis as downstream effect enzyme and induces functional proteins cracking and apoptosis [27,28]. In this study we found that Caspase-3 and NF-kB had high expression near the hemorrhage even it had been removed for weeks. It implied that the injury would sustain after the therapy. While As the mechanism of delay injury is complicated, we would continue to study the mechanism and find a better way to reduce the delay injury, for improving the quality life of patients.
Acknowledgement
This study was supported by Youth Project of National Natural fund (NO: 81400648), Beijing Scientific and Technologic Supernova Supportive Project (NO: 15111000030000/ XXJH2015B100), Youth Culture Project of Chinese PLA (NO: 13QNP171), Surface Project of Hainan Province Natural Fund (NO: 20158315) and Clinical Advantage Supportive Programs of Chinese PLA General Hospital (NO: 2012FC-TSYS-4028).
References
- Navi BB, Parikh NS, Lerario MP. Risk of Intracerebral Hemorrhage after Emergency Department Discharges for Hypertension. J Stroke Cerebrovascular Dis 2016.
- Szymanska A, Biernaskie J, Laidley D, Granter-Button S,Corbett D. Minocycline and intracerebral hemorrhage: influence of injury severity and delay to treatment. ExpNeurology 2006;197:189-96.
- Keigler G, Goldberg I, Eichel R, GomoriJM,Cohen JE, Leker RR. Diffusion-weighted imaging at b1000 for identifying intracerebral hemorrhage: preliminary sensitivity, specificity, and inter-rater variability. J Stroke Cerebrovascular Dis 2014;23:1934-1938.
- Gioia LC, Kate M, McCourt R. Perihematoma Cerebral blood flow is unaffected by statin use in acute intracerebral hemorrhage patients. J Cerebral Blood Flow Metabol 2015; 35: 1175-1180.
- Naganuma T, Takemoto Y, Shoji T, Ishimura E, Okamura M, Nakatani T. Cerebral Microbleeds Predict Intracerebral Hemorrhage in Hemodialysis Patients. Stroke J Cerebral Circulation 2015; 46: 2107-2012.
- Fan CH, Liu HL, Huang CY, Ma YJ, Yen TC, Yeh CK. Detection of intracerebral hemorrhage and transient blood-supply shortage in focused-ultrasound-induced blood-brain barrier disruption by ultrasound imaging. Ultrasound Med Biol 2012; 38: 1372-1382.
- Mun-Bryce S, Wilkerson AC, Papuashvili N, Okada YC. Recurring episodes of spreading depression are spontaneously elicited by an intracerebral hemorrhage in the swine. Brain Res 2001; 888: 248-255.
- Desland FA, Afzal A, Warraich Z, Mocco J. Manual versus Automated Rodent Behavioral Assessment: Comparing Efficacy and Ease of Bederson and Garcia Neurological Deficit Scores to an Open Field Video-Tracking System. J Central Nervous Syst Dis 2014; 6: 7-14.
- Xia X, Cheng G, Pan Y, Xia ZH, Kong LD. Behavioral, neurochemical and neuroendocrine effects of the ethanolic extract from Curcuma longa L. in the mouse forced swimming test. J Ethnopharmacol 2007; 110: 356-363.
- Curzon P, Zhang M, Radek RJ, Fox GB. The Behavioral Assessment of Sensorimotor Processes in the Mouse: Acoustic Startle, Sensory Gating, Locomotor Activity, Rotarod, and Beam Walking. In: Buccafusco JJ, ed. Methods of Behavior Analysis in Neuroscience. 2nd Ed. Boca Raton (FL) 2009.
- He Q, Bao L, Zimering J, Zan K, Zhang Z. The protective role of (-)-epigallocatechin-3-gallate in thrombin-induced neuronal cell apoptosis and JNK-MAPK activation. Neuroreport 2015; 26: 416-423.
- Liu XC, Jing LY, Yang MF. Enhanced Neuroprotection of Minimally Invasive Surgery Joint LocalCooling Lavage against ICH-induced Inflammation Injury and Apoptosis in Rats. Cell MolNeurobiol 2016; 36: 647-655.
- MacLellan CL, Silasi G, Poon CC. Intracerebral hemorrhage models in rat:Comparing Collagenase to blood infusion. J Cerebral Blood Flow Metabol 2008; 28: 516-525.
- Tanoue S, Inamasu J, Yamada M, Toyama H, Hirose Y. Does dabigatran increase the risk of delayed hematoma expansion in a rat model of Collagenase-induced intracerebral hemorrhage? Journal Stroke Cerebrovascular Dis 2015; 24: 374-380.
- Chen JC. The effects of acupuncture and traditional Chinese medicines on apoptosis of brain tissue in a rat intracerebral hemorrhage model. PhysiolBehav 2015; 151: 421-425.
- Platz J, Güresir E, Wagner M, Seifert V, Konczalla J. Increased risk of delayed cerebral ischemia in subarachnoid hemorrhage patients with additional intracerebral hematoma. J Neurosurg 2016.
- John RF, Colbourne F. Delayed localized hypothermia reduces intracranial pressure following collagenase-induced intracerebral hemorrhage in rat. Brain Res 2016; 1633: 27-36.
- Guresir E, Schuss P, Borger V, Vatter H. Experimental subarachnoid hemorrhage: double Cisterna magna injection rat model-assessment of delayed pathological effects ofCerebral vasospasm. Transl Stroke Res 2015; 6: 242-251.
- Liu SN, Sun SJ, Liu Q, Hou SC, Shen ZF. Effect of Mudan Granule on islets beta Cell function in monosodium glutamate induced obese mice with insulin resistance: an experimental study. ZhongguoZhong xi yijie he zazhiZhongguoZhongxiyijiehezazhi 2014; 34: 853-858.
- Yang N, Farrell A, Niedelman W. Genetic basis for phenotypic differences between different Toxoplasma gondii type I strains. BMC Genomics 2013; 14: 467.
- Zuo L, Shi L, Yan F. The reciprocal interaction of sympathetic nervous system and cAMP-PKA-NF-kB pathway in immune suppression after experimental stroke. NeurosciLett 2016; 627: 205-210.
- Hu Y, Ge W, Wang X, Sutendra G. Caspase cleavage of iASPP potentiates its ability to inhibit p53 and NF-kB. Oncotarget 2015; 6: 42478-42490.
- Becatti M, Prignano F, Fiorillo C, Pescitelli L, Nassi P. The involvement of Smac/DIABLO, p53, NF-kB, and MAPK pathways in apoptosis of keratinocytes from perilesionalvitiligo skin: Protective effects of curcumin and capsaicin. Antioxid Redox Signal 2010; 13: 1309-1321.
- Goval JJ, ThielenC, BourguignonC. The prevention of spontaneous apoptosis of follicular lymphoma BCells by a follicular dendriticCell line: involvement ofCaspase-3,Caspase-8 andC-FLIP. Haematologica 2008;93:1169-1177.
- Zhou H, Zhang H, Yan Z, Xu R. Transplantation of human amniotic mesenchymal stemCells promotes neurological recovery in an intracerebral hemorrhage rat model. BiochemBiophysical ResCommunications 2016;475:202-208.
- Lu H, Jiang M, Lu L, Zheng G, Dong Q. Ultrastructural mitochondriaChanges in perihematomal brain and neuroprotective effects of Huperzine A after acute intracerebral hemorrhage. Neuropsychiatr Dis Treat 2015;11:2649-2657.
- Liu G, Wang T, Wang T, Song J, Zhou Z. Effects of apoptosis-related proteins caspase-3, Bax and Bcl-2 on cerebral ischemia rats. Biomed Rep 2013; 1: 861-867.
- Assefa Z, Bultynck G, Szlufcik K.Caspase-3-induced truncation of type 1 inositol trisphosphate receptor accelerates apoptoticCell death and induces inositol trisphosphate-independentCalcium release during apoptosis. J BiolChem 2004;279:43227-43236.