Research Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2017) Volume 7, Issue 60
Tumor Necrosis Factor (TNF-?)-Induced Upregulation of Cartilage Degradation-Associated Genes by Chondrocytes Requires Epithelial Sodium Channel Activity
Min Li1, Ge Li2, Lu Tang1, Xingyan Lu1, Jun Chen1, Qingnan Li1*, Guozhu Yang1, Huanhuan Jia2, Yu’e Wu2 and Yu Zhang2
1Department of Life Science, Guangdong Pharmaceutical University, Guangzhou, Guangdong Province, P.R.China
2Guangdong Laboratory Animals Monitoring Institute, Guangzhou, Guangdong Province, P.R.China
- *Corresponding Author:
- Qingnan Li
Department of Life Science
Guangdong Pharmaceutical University
Guangzhou, Guangdong Province, P.R.China
E-mail: qingnanli@sina.com
Accepted on February 28, 2017
Abstract
Purpose: Chronic inflammation due to increased TNF-α is the main cause of Rheumatoid Arthritis cartilage destruction. We investigated the effects of hTNF-α on chondrocyte and its relationship with water salt metabolism.
Methods: Chondrocytes were cultured from the knee cartilage of ICR mouse pups. Various concentrations of hTNF-α (0, 25, 50, 100 ng/ml) were used to treat chondrocytes and apoptosis was measured by flow cytometry. hTNF-α 25 ng/ml was used in TNF-α, Amiloride (Ami), TNF-α+Ami and control groups. MMPs, IL-1α, BAX and caspase 3 mRNA levels were examined by Real-time PCR. The length and growth rate of metatarsus were measured by stereo microscope. Histological changes were observed by H&E staining and Toluidine blue staining. TUNEL staining determined the cartilage apoptosis on the tissue level.
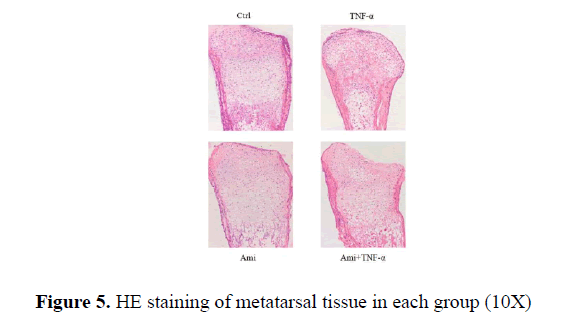
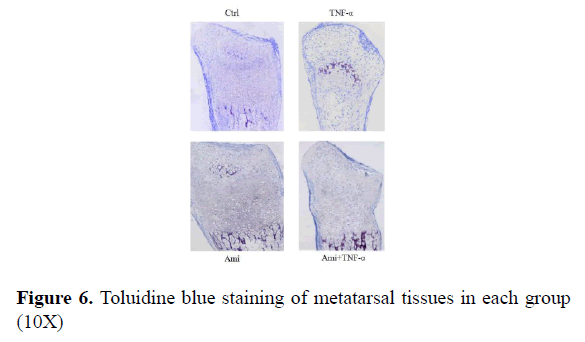
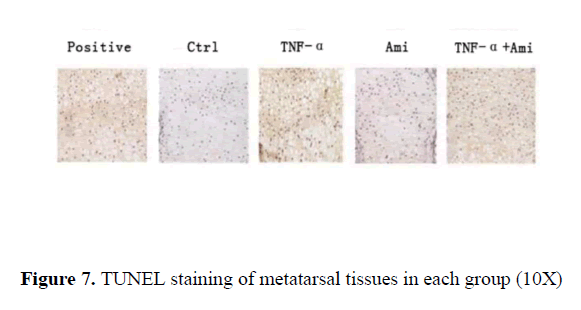
Results: Our result further conformed that hTNF-αin three concentrations significantly enhanced apoptosis compared to the control group (p<0.05). Also hTNF-α significantly increased MMP9, MMP13, IL-1α, BAX and caspase 3 mRNA expression (p<0.05) compared with control group, and added Ami on hTNF-αgroup can reduce the above indicators and partially neutralized the effect of hTNF-α on MMP9, MMP13, IL-1α (p<0.05) and almost neutralized the effect of hTNF-α on BAX and caspase 3 in vitro. In metatarsals ex vivo, TNF-α inhibited metatarsal growth (P<0.01) while Ami had no significant effect compared to the control group. However, metatarsal growth rate in Ami+TNF-α group was lower than control and higher than TNF-α-treated group (P<0.01). HE and Toluidine blue staining showed that the proliferative area of chondrocytes in hTNF-α group was significantly decreased with chaotic alignment in chondrocyte layer. Ami showed no changes and hTNF-α+Ami treatment showed some improvement compared to hTNF-α group but was worse than Ami group. TUNEL staining showed that hTNF-α led to significant chondrocytic apoptosis while Ami did not compared to the control. hTNF-α+Amiloride showed milder apoptosis compared to hTNF-α group.
Conclusion: In Summary, hTNF-αimpacts chondrocytes by promoting MMPs and apoptosis. Amiloride could partially neutralize the above indicators in vitro and metatarsals ex vivo. It was speculated that there existed a relation in the effect of TNFa on chondrocytes with ENaC, a key protein in water salt metabolism.
Keywords
Rheumatoid arthritis, Chondrocytes; TNF-α, Epithelial sodium channel, Apoptosis
Introduction
Tumor necrosis factor alpha (TNF-α) is an important inflammatory marker which play an important role in RA pathogenesis and results in synovitis. Our previous studies have shown that TNF-α-overexpressing mice lead to the destruction of articular cartilage [1].
The process of secretion and degradation of matrix by chondrocytes maintain a dynamic homeostasis in normal physical state and causes diseases in abnormal conditions, such as rheumatoid arthritis, degenerative arthritis [2]. The self-repairing and proliferation ability of chondrocytes is poor. The eroding degeneration of articular cartilage will aggravate in the event of apoptosis caused by some form of stimulus.
It was found in the late 1990s that Epithelial Sodium Channel (ENaC) exists in epithelial cells but also in chondrocytes [3,4]. ENaC was a non-voltage-dependent ion channel. It is also a key protein for water salt metabolism, and has an indispensable role in the growth, metabolism and energy transformation of the organism [5-8]. ENaC is an amiloride Ami-sensitive sodium channel. Ami can inactivate ENaC or suppress its expression resulting in the exertion of the biological function preventing Na+ entering the cells [9,10].
TNF-α leads to the destruction of articular cartilage and chondrocytes, while chondrocytes express ENaC. We used chondrocytes and metatarsi as models, to address there exist relationship between TNFa affects chondrocyte involvement through ENaC, and also explore its mechanism.
Materials and Methods
0.25% w/v trypsin, 0.02% EDTA, collagenase II (0.2%), Dulbecco's modified Eagle's medium(DMEM)-high glucose,fetal bovine syndrome(FBS) (Hyclone), 100-100U/ml penicillin-streptomycin (Gibco), ascorbic acid-2- phosphate(Sigma), RNAiso reagent (TaKaRa), Annexin V and Propidium Iodide Double Staining reagent, TUNEL staining reagent and other reagents.
Flow cytometry (BD Biosciences), real-time PCR system (ABI, 7500 ordinary type), automatic slicer (Leica) and other instruments.
Cell cultures
Newborn ICR mice were purchased from Guangzhou University of Traditional Chinese Medicine (TCM). The costal cartilage was dissociated by a 30 min treatment with trypsin/ EDTA (0.25% w/v trypsin, 0.02% EDTA) followed by a 4 h digestion in collagenase II (0.2%) at 37°C. All procedures conformed to the guidelines of the Animal Care and Use Committee of Guangdong Pharmaceutical University. The cells were expanded in a humidified atmosphere containing 95% air and 5% CO2 at 37°C. Cells from the first and second passages were cultured in basal medium composed of Dulbecco's modified Eagle's medium (DMEM)-high glucose, 10% fetal bovine syndrome (FBS) (Hyclone), 100-100 U/ml penicillin-streptomycin (Gibco), and 50 ug/ml L ascorbic acid-2- phosphate (Sigma). Cells at passages 3-5 were used for the following studies.
Annexin v and propidium iodide double staining by flow cytometry
Cell apoptosis was measured by flow cytometry of annexin V/ propidium iodide (PI) double-stained cells. Cells were treated with various concentrations of hTNF-α (0, 25, 50, or 100 ng/ ml), deplated with trypsin, washed with 1 mL phosphate buffered saline (PBS), suspended in binding buffer (10 mM HEPES/NaOH, 140 mM NaCl, 2.5 mM CaCl2, pH 7.4), and stained with 5 μL of FITC-conjugated Annexin V and 5 μL of PI (both from 50 mg/mL stocks). The mixture was incubated for 15 min at room temperature in the dark and analyzed by flow cytometry (BD Biosciences).
RT-qPCR analysis
RNA Extraction and cDNA Synthesis: Total RNA was extracted using the RNAiso reagent (TaKaRa) according to the manufacturer’s instructions. Potentially contaminating DNA was eliminated from total RNA with RNase-free DNaseI (TaKaRa). Only RNA samples with an optical density absorption ratio A260/A280 of 1.8-2.0 and an A260/A230 ratio >2.0 were used for subsequent analysis. RNA purity was assessed on a BioPhotometer D30 (Eppendorf). First-strand cDNA was synthesized with the M-MLV (RNase H−) (TaKaRa, Japan) and oligo-dT primers.
RT-qPCR: The RT-qPCR reactions were run on a Mastercycler ep realplex real-time PCR system (ABI, 7500 ordinary type) with SYBR Premix Ex Taq II (Perfect Real Time, TaKaRa). Each reaction was performed in a 20 μl volume containing 80 ng of each cDNA, 200 nM of each primer, and 10 μl SYBR Premix. The following amplification program was used: initial denaturation at 95°C for 30 s, then 40 cycles of 95°C for 5 s and 60°C for 34 s. Mean Ct values were calculated.
Primer design: Primers (Table 1) were designed and synthesized by Sangon Biotechnology (Shanghai, China).
| Gene | Primer sequences (forward/reverse) | Size (bp) |
|---|---|---|
| MMP-13 | 5'-GTGTGGAGTTATGATGATGT-3' | 141 |
| 5'-TGCGATTACTCCAGATACTG-3' | ||
| Bax | 5'-GAACAGATCATGAAGACAGGG-3' | 162 |
| 5'-CAGTTCATCTCCAATTCGCC-3' | ||
| MMP-9 | 5'-CTGGCGTGTGAGTTTCCAA-3' | 130 |
| 5'-CGGTTGAAGCAAAGAAGGAG-3' | ||
| IL-1a | 5'-CAGTATCAGCAACGTCAAGCA-3' | 124 |
| 5'-CTGGGTTGGATGGTCTCTTC-3' | ||
| GAPDH | 5'-TGTAGGCCAGGTGATGCAAG-3' | 130 |
| 5'-TACGCATTATGCCCGAGGAC-3' | ||
| Caspase3 | 5'-GAGCCAGAGCAGAGACTTGG-3' | 129 |
| 5'-CGTGGTAACTTGGACATCATCC-3' |
Table 1. Primer sequences and amplicon characteristics for each of the fifteen reference genes
Metatarsal culture
Timed pregnant ICR mice were sacrificed for isolation of embryonic metatarsals. All animals were housed in a virus-and parasite-free barrier facility under 12 h light/12 h dark cycles with free access to food and water.
Suckling mice disinfection with 75% (by volume) ethanol, we use continuous zoom stereo microscope to take metatarsal, then moved to a 24-well culture plates after washing with PBS, added 200 μL drug-free basal medium, 37, 5% (volume fraction) of carbon dioxide in the culture for 72 h. After 72 h, metatarsal attached to the bottom of the culture plate.
Metatarsal length measurement
After metatarsal attached to the rear, we take photo for the first time to measure (stereo microscope comes with measurement software) the length of each set of each metatarsal, calculate the growth rate, the formula is: growth rate %=(a mean value of the first-the first time the average value)/the first average X100%, a>1, n=5. After the first measurement, drug-containing medium was changed after a measurement every three days, 10 repetitions.
Serial midsagittal sections (4 μm-thick) were cut from paraffin-embedded, decalcified metatarsals tissue, blocks using a microtome. Sections were stained with hematoxylin and eosin (H&E) and toluidine blue for histological assessment. TUNEL staining was used for the detection of dead chondrocytes.
Statistical analysis
Statistical analysis was performed using SPSS software, version 11.0 (SPSS, Chicago, IL, USA). All data acquisition and analysis was performed blindly. Quantitative data for control and experimental groups were subjected to one-way ANOVA and Student-Newman-Keuls (SNK-q) post-test. P-values of 0.05 were considered to be statistically significant.
Ethics statement
The study was approved by the research ethics committee of Guangdong Pharmaceutical University. All animals care and experimentation were approved by the experimental animal centre of Guangdong Pharmaceutical University. Efforts were made to reduce the number of animals used and minimize animal suffering.
Results
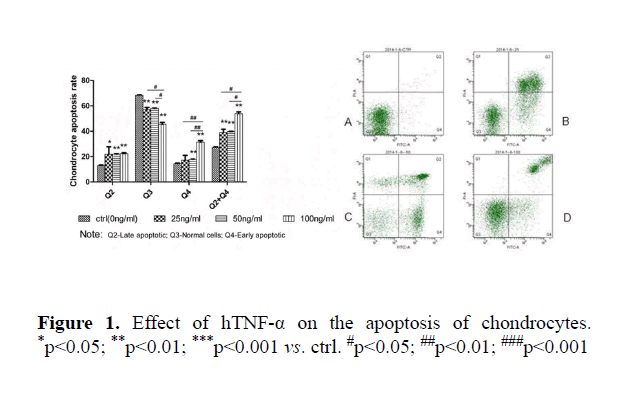
Effect of hTNF-α with different concentrations on apoptosis of mouse chondrocytes
Compared to the control group, all concentrations of TNF-α significantly increased the apoptosis of chondrocytes (ctrl: Q2 12%, Q3 68%, Q4 14%, 25 ng/ml: Q2 22%, Q3 56%, Q4 16%, 50 ng/ml: Q2 23%, Q3 57%, Q4 18%, 100 ng/ml: Q2 24%, Q3 44%, Q4 30%) at both early and late stages (p<0.05). The sum of apoptotic rate at early and late stages was increased correspondingly. No dose-dependent effect was observed (Figures 1 and 2).
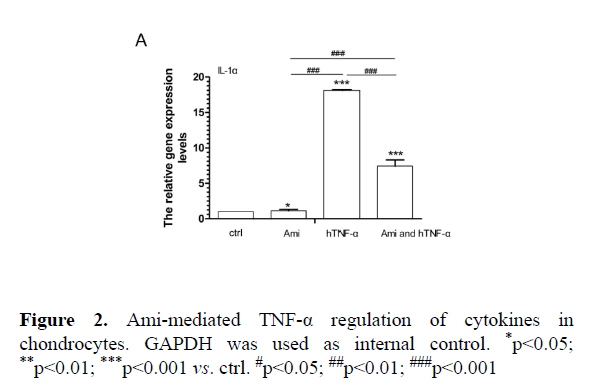
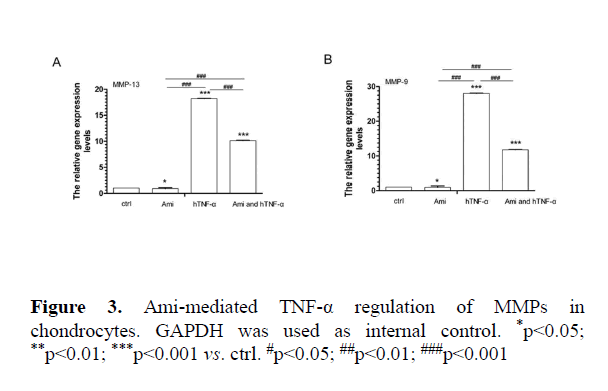
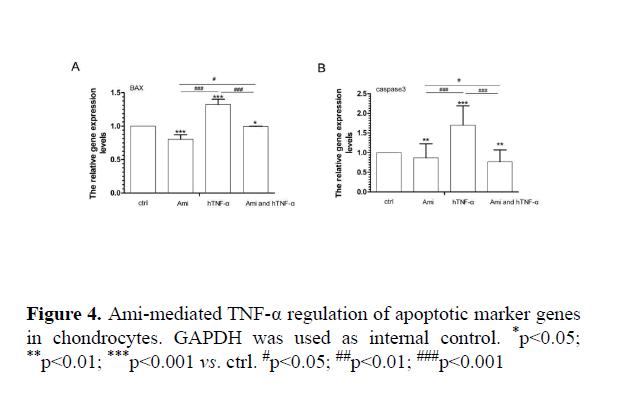
Effects of AMI on MMPs, cytokines and apoptotic marker gene expression in chondrocytic cells
AMI intervention with hTNF-α in cell-based assay: Compared to the control group, hTNF-α significantly increased MMP9, MMP13, IL-1α, BAX and caspase3 expression while Ami reduced MMP13, MMP9, IL-1α, BAX and caspase 3 mRNA levels. hTNF-α+ Ami treatment partially neutralized the effect of hTNF-α (p<0.05, Figures 3 and 4).
Effects of Ami on chondrocytic maturation in metatarsals ex vivo
Compared to the control group, TNF-α inhibited metatarsal growth (P<0.01) while Ami had no significant effect. However, metatarsal growth rate in Ami+TNF-α group was lower than control and higher than TNF-α-treated group (P<0.01, Table 2).
| Time(d) | Ctrl | Ami (20uM) | TNF-a (25ng/ml) | Ami+TNF-a | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD(mm) | Growth rate(%) | Mean ± SD(mm) | Growth rate(%) | Mean ± SD(mm) | Growth rate(%) | Mean ± SD(mm) | Growth rate(%) | |
| 0 | 2.22 ± 0.149 | 2.21 ± 0.046 | 2.00 ± 0.090 | 2.20 ± 0.122 | ||||
| 12 | 2.36 ± 0.244 | 6.58 | 2.44 ± 0.020 | 10.56 | 2.21 ± 0.208 | 10.32 | 2.24 ± 0.140 | 5.47 |
| 18 | 2.40 ± 0.225 | 8.08 | 2.52 ± 0.055 | 13.88 | 2.19 ± 0.226 | 9.32 | 2.26 ± 0.077 | 2.96 |
| 24 | 2.41 ± 0.181 | 8.83 | 2.52 ± 0.068 | 14.18 | 2.08 ± 0.204** | 3.83 | 2.23 ± 0.091** | 2.39 |
| 30 | 2.47 ± 0.115 | 11.24 | 2.50 ± 0.060 | 13.12 | 2.05 ± 0.234** | 2.5 | 2.29 ± 0.116** | 4.32 |
Table 2. Ami-mediated TNF-a regulation of chondrocytic growth in cultured metatarsals
Compared to the control group, TNF-α significantly suppressed metatarsal growth while Ami had no significant effect. Metatarsal growth in Ami+TNF-α group was higher than that in hTNF-α group but lower than that in Ami. The difference was statistically significantp<0.01.
Effect of Ami on HE staining in cultured in metatarsals ex vivo
HE and Toluidine blue staining showed that the proliferative area of chondrocytes in hTNF-α group was significantly decreased with chaotic alignment in chondrocyte layer. Ami showed no changes and hTNF-α+ Ami treatment showed some improvement compared to hTNF-α group but was worse than Ami group. TUNEL staining showed that compared to the control, hTNF-α led to significant chondrocytic apoptosis while Ami did not (Figure 5).
Effect of Ami on Alcian blue staining in metatarsals ex vivo
From Figure 6 we could see that in the control group there were many chondrocytes in the proliferative area with clear alignment; in comparison, there were fewer chondrocytes in TNF-α-treated group and cell alignment can be barely seen; Ami group showed fewer changes compared to the control. TNF-α+ Ami group was between control and TNF-α group. Where there was some improvement but cell alignment was not clear enough (Figure 6).
Effect of Ami on TUNEL staining in metatarsals ex vivo
The positive control treated by DNase where DNA breakage was stained as brown particles suggested excellent slide processing. In comparison, almost no brown particles were observed in the control. Similar results were seen in Ami group. In contrast, clear brown particles were observed in TNF-α treated group while in TNF-α+Ami group, fewer brown particles were seen compared to TNF-α group (Figure 7).
Discussions
Previous report published by our research team pointed out that TNF-α could downregulate chondrocyte growth related genes, upregulates matrix-degrading enzyme, apoptosis related gene and ENaC-α gene [11].
ENaC belongs to the ENaC/DEG superfamily which is a type of glycosylated membrane protein that regulates the transmembrane transport of Na+ and the homeostasis of Na+. ENaC is a novel non-voltage-dependent sodium channel and is the rate-limiting step of sodium reuptake, which can be blocked by its specific inhibitor Ami.
In this study, we applied flow cytometry to further confirm that TNF-α induced chondrocytic apoptosis. Furthermorewe utilized ENaC inhibitor, Ami together with TNF-α to examine the expression of chondrocyte-associated MMPs, IL-1α, apoptotic marker, BAX and caspase 3. It was showed that TNF-α stimulated chondrocytes to secret MMPs and cytokines as well as promoted the expression of apoptotic genes. Ami partially neutralized the effect of TNF-α on matrix degradation and chondrocytic apoptosis in vitro, which was similar with the observation that Ami could block TNF-α-induced increase of water sodium flux in lung [12], suggesting that ENaC-α might be participate in TNF-α mediated disruption of chondrocytes.
To further validate the hypothesis, we performed ex vivo metatarsal culture. During the fetal development and infancy, the majority of skeleton is made of cartilage where chondrocytes are very active. Therefore, ex vivo metatarsal culture is a good model to study chondrocytic proliferation, differentiation and apoptosis [13-17]. Phenotypically, we measured the length of metatarsus over time and the results showed that TNFa co-treatment of Ami could partially prevent the TNF-α-mediated reduction of growth rate over time. Microcosmically, we prepared metatarsal tissue sections of each group for staining. H&E staining showed proliferative area and chondrocytes in TNF-α+ Ami group was between those in the TNF-α and Ami alone groups. TUNEL staining showed that TNF-α induced apoptosis was suppressed by Ami and that Ami partially neutralized the stimulatory effect of TNF-α on chondrocytic apoptosis.
The potential mechanisms underlying the above changes can be explained as follows: from extracellular matrix level, TNF-α promoted the secretion of MMP-9 and MMP-13 and resulted in excessive matrix degradation. The loss of microenvironment that was essential for chondrocyte survival led to decreased proliferative area of chondrocytes and slower growth of metatarsals; from the cellular level, TNF-α induced chondrocytic apoptosis which depleted the seeding cells of cartilage and correspondingly, the secretion of matrix was also impaired, leading to irregular alignment of chondrocytes in the proliferative area and decreased growth rate. These changes could be partially neutralized by ENaC inhibitor, Ami as indicated by histology results, suggesting the involvement of ENaC and the relationship between water salt metabolism and TNF-α.
Although the role of Na+ in cartilage remains unclear, it has been reported that Na+ is often located at the frontline of bone mineralization [18]. ENaC has been found on chondrocytes. Based on our findings, we speculated that ENaC on the chondrocytes could regulate Na+ transport in such microenvironment, which was consistent with the findings in lung [11]. It has been reported [19] that there was Na+ permeable channel proteins on the type II epithelial cell membranes in alveolus.
In summary, our data suggested that TNF-α regulation of chondrocytes adjust bone development might mediate by ENaC, and that indicates water salt metabolism was associated with bone metabolism.
It was reported the expression of ENaC and Na+-k+ ATP enzyme on chondrocytes and was present [20]. Na+-k + ATP enzyme pump was not studied in this study. The mechanism in the protein level was yet to be investigated in this study and further research is needed.
Acknowledgments
This work was supported by the planned Science and Technology Project of Guangdong Province, China (2014A030304029).
References
- Li G, Wu Y, Jia H, Tang L, Huang R, Peng Y, Zhang Y. Establishment and evaluation of a transgenic mouse model of arthritis induced by overexpressing human tumor necrosis factor alpha. Biol Open. 2016; 5: 418-423.
- Gong JH, Li JN. Progress of research on cartilage injury and repair in osteoarthritis. Orthop J China. 2006: 941-44.
- Trujillo E, Alvarez de la Rosa D, Mobasheri A, González T, Canessa CM, Martín-Vasallo P. Sodium transport systems in human chondrocytes. II. Expression of ENaC, Na+/K+/2Cl-cotransporter and Na+/H+ exchangers in healthy and arthritic chondrocytes. Histol Histopathol. 1999; 14: 1023-1031
- Mobasheri A. Regulation of Na+, K+-ATPase density by the extracellular ionic and osmotic environment in bovine articular chondrocytes. Physiol Res. 1999; 48: 509-512
- Heaney RP. Role of dietary sodium in osteoporosis. J Am Coll Nutr. 2006; 25: 271S-276S.
- JC Aub, DM Tibbetts, R Mclean. The influence of parathyroid hormone, urea, sodium chloride, fat and of intestinal activity upon calcium balance. J Nutr. 1937; 113:635–655.
- Shortt C, Flynn A. Sodium-calcium inter-relationships with specific reference to osteoporosis. Nutr Res Rev. 1990; 3: 101-115.
- Goulding A, Campbell D. Dietary NaCl loads promote calciuria and bone loss in adult oophorectomized rats consuming a low calcium diet. J Nutr. 1983; 113: 1409-1414.
- JG Huang, HJ Tong, HQ Liu, XL Zhang. Effects of IL-1ß and TNF-a on degradation of extracellular matrix of articular chondrocytes and related mechanism. J Shanghai Jiaotong University. 2010; 30: 1084-1089.
- Zwerina J, Redlich K, Polzer K, Joosten L, Krönke G, Distler J, Hess A, Pundt N, Pap T, Hoffmann O, Gasser J, Scheinecker C, Smolen JS, van den Berg W, SchettG. TNF-induced structural joint damage is mediated by IL-1. Proc Natl Acad Sci. 2007; 104: 11742-11747.LI Min, LI Ge, JIA Huanhuan, WU Yue, TANG Lu, LU Xingyan. Effects of different concentrations hTNFa on chondrocytes and matrix-associated gene expression. Chinese J Osteoporos. 2015; 21;541-545
- Pu Y, Luo KQ, Chang DC. A Ca2+ signal is found upstream of cytochrome c release during apoptosis in HeLa cells. Biochem Biophys Res Commun. 2002; 299: 762-769.
- Vikram S. Chhokar, Yao Sun, Syamal K. Bhattacharya, Robert A. Ahokas, Linda K. Myers, Zhiqing Xing,et al. Loss of bone minerals and strength in rats with aldosteronism. Am J Physiol Heart Circ Physiol. 2004; 287: H2023-H2026
- Zalutskaya AA, Cox MK, Demay MB. Phosphate regulates embryonic endochondral bone development[J].J Cell Biochem. 2009; 108: 668-74.
- Koshimizu T, Kawai M, Kondou H, Tachikawa K, Sakai N, Ozono K, Michigami T. Vinculin functions as regulator of chondrogenesis. J Biol Chem. 2012; 287: 15760-15775.
- Ionescu A, Kozhemyakina E, Nicolae C, Kaestner KH, Olsen BR, Lassar AB. FoxA family members are crucial regulators of the hypertrophic chondrocyte differentiation program. Dev Cell. 2012; 22: 927-39.
- Cooper KL, Oh S, Sung Y, Dasari RR, Kirschner MW, Tabin CJ. Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions. Nature. 2013; 495: 375-78.
- Kuhne M, John T, El-Sayed K, Marzahn U, Aue A, Kohl B, Stoelzel K, Ertel W, Blottner D, Haisch A, Schulze-Tanzil G. Characterization of auricular chondrocytes and auricular/articular chondrocyte co-cultures in terms of an application in articular cartilage repair. Int J Mol Med. 2010; 25: 701-8.
- HE Lin. Signal transduetion study of sodium transport in alveolar type?Epithelial cells of ARDS rats [D]. Southern Medical University, 2008.
- Kamkin A, IKiseleva I. Enigmatic roles of the epithelial sodium channel (ENaC) in articular chondrocytes and osteoblasts: Mechanotransduction, sodium transport or extracellular sodium sensing Mechanosensitivityin Cells and Tissues ed by Andre Kamkin & Irina Kiseleva. Academia Moscow. 2005: 452-464