- Biomedical Research (2010) Volume 21, Issue 3
Tryptophan and the kynurenine pathway in haemodialysis and peritoneal dialysis
Bipath Priyesh and Viljoen* MargarethaDepartment of Physiology, School of Medicine, Faculty of Health Sciences, University of Pretoria, Pretoria, South African
- Corresponding Author:
- M. Viljoen
Department of Physiology- Medicine
University of Pretoria
PO Box 2034
Pretoria, South African 0001
Accepted date: April 09 2010
Abstract
Tryptophan is an essential amino acid necessary for the synthesis of proteins, serotonin and niacin. Main causes of tryptophan depletion are malnutrition and pro-inflammatory activity. Kynurenine and quinolinic acid, metabolites of the kynurenine pathway, may accumulate in end stage renal disease (ESRD) patients on renal replacement therapy. Objective of the study is to simultaneously measure tryptophan and kynurenine pathway metabolites and to compare the levels found in haemodialysis to that of peritoneal dialysis patients. Tryptophan, kynurenine and quinolinic acid levels, in blood from 30 ESRD, i.e. 15 haemodialysis and 15 peritoneal dialysis patients, were quantified by gas chromatography – mass spectrometry. Tryptophan was significantly (p<0.05) lower for both haemodialysis (5.3 ± 5.0 μmol/L) and peritoneal dialysis (6.7 ± 3.2 μmol/L) groups than for controls (28.4 ± 4.3 μmol/L). Kynurenine was significantly (p<0.05) higher in the haemodialysis (4.7 ± 1.9 μmol/L) versus control group (2.1 ± 0.6 μmol/L). Quinolinic acid was significantly (p<0.05) higher in both the haemodialysis (4.9 ± 2.03 μmol/L) and peritoneal dialysis (2.8 ± 2.03 μmol/L) groups than in controls (0.3 ± 0.15 μmol/L). There were no differences between the patient groups other than kynurenine being significantly (p<0.05) lower in the peritoneal dialysis (2.9 ± 0.8 μmol/L) group. Patient tryptophan levels were lower than that reported by European studies. Depletion of tryptophan and accumulation of kynurenine and quinolinic acid occur in HD and PD patients. Differences in nutritional status and infection rates may contribute to greater tryptophan depletion in developing countries than in Europe and should be further investigated.
Keywords
tryptophan, kynurenine, renal disease, haemodialysis, peritoneal dialysis
Introduction
End stage renal disease (ESRD) is characterized by accumulation of uraemic toxins as a result of inadequate clearance by the failing kidneys. These so-called uraemic toxins have the potential to negatively influence the individual. Many symptoms of ESRD, such as skeletal abnormalities, pruritus, neurological abnormalities, temperature disturbances, vomiting, immunological disturbances, anaemia, cardiovascular problems and increases in the formation of reactive oxidative species have been ascribed, at least partially, to an excess of one or more of these substances [1]. At least 90 substances have been identified by the European Uraemic Toxin Work Group as uraemic toxins [2]. In ESRD patients on renal replacement therapies such as haemodialysis and peritoneal dialysis these molecules are removed at different rates, not only depending on their molecular weights and the characteristics of the dialysis membranes, but also on the length of the dialysis sessions.
In addition to the accumulation of many substances in the blood of ESRD patients on renal replacement therapies, these patients also often suffer from malnutrition [3,4,5,6]. Reports on the frequency of malnutrition at different dialysis centres vary, probably due to the differences in the populations and the type of dialysis, but possibly also as a result of the way malnutrition is assessed. Depending on the studies between 18 and 50 percent of patients on peritoneal dialysis and between 23 and 76 percent of haemodialysis patients are reported to suffer from malnutrition [3,7,8,9,10]. Malnutrition is, however, not limited to dialysis patients but often also occurs in the pre-dialysis stages [11]. Estimation of nutritional status has always been, and still is problematic. Many different types of assessments ranging from the physical characteristics of the individual, to bio-electrical assessments, to a variety of biochemical markers, have been used. However, albumin level, although not ideal, remains the most commonly used indicator of malnutrition [3,12]. The malnutrition of chronic renal failure patients is generally thought to be multifactorial and factors known to contribute include low protein-energy intake due to prescribed diets, uraemia-suppressed appetites, metabolic acidosis, endocrine disturbances, loss during the dialysis process and others [3,13]. Another contributing factor to the low albumin in the blood of ESRD patients, and probably that of other nutritional factors, especially in patients on dialysis, is pro-inflammatory activity [14,15,16]. It is highly likely that the pro-inflammatory condition in ESRD patients, especially those on haemodialysis and peritoneal dialysis, may contribute not only to the state of malnutrition, but also to the accumulation of uraemic toxins as will be discussed later.
A very important substance of which, in ESRD patients, the levels may be influenced by both diet and proinflammatory activity is the essential amino acid tryptophan. Tryptophan is transported in blood, either as unconjugated tryptophan or as a protein bound complex. In the tissues it can follow one of several pathways under the influence of specific enzymes. One of the most important functions of tryptophan is its utilization for the synthesis of tissue proteins [17,18]. Other important pathways of tryptophan metabolism include those for the production of serotonin in the nervous system and other body cells, the production of melatonin in the pineal gland, and metabolism along the kynurenine pathway [17,19]. The majority of tryptophan is metabolised along the kynurenine pathway which results in the biosynthesis of nicotinamide adenine dinucleotide and complete oxidation of tryptophan [17,20]. The metabolism of tryptophan along the kynurenine pathway is under the influence of the two enzymes tryptophan 2,3 dioxygenase (TDO) and indoleamine 2,3 dioxygenase (IDO) [21,22]. IDO is induced by pro-inflammatory cytokine activity [17,22]. This not only results in tryptophan degradation but also in accumulation of kynurenine, quinolinic acid and kynurenic acid. These metabolites of the kynurenine pathway are now seen to form part of the protein-bound uraemic toxin load of ESRD patients [2].
There are indications that ESRD patients on dialysis are tryptophan depleted [23] and that an accumulation of kynurenine pathway metabolites, such as quinolinic acid, may contribute to certain uraemic symptoms such as anaemia [24], certain neurological disturbances and increased vulnerability to infections [21,25]. A need exists to simultaneously determine the levels of tryptophan and metabolites of the kynurenine pathway and to compare the effects of the two major treatment modalities, i.e. haemodialysis and peritoneal dialysis, on their levels.
The aim of this study was to simultaneously measure tryptophan and kynurenine pathway metabolites and to compare the levels found in haemodialysis to that of peritoneal dialysis patients.
Materials and Methods
Study Groups
The study population consisted of 30 end-stage chronic renal failure patients on haemodialysis (HD) and peritoneal dialysis (PD) treatment. The haemodialysis group consisted of 15 end-stage chronic renal failure patients with a mean (mean ± SD) dialysis period of 278.4 ± 266.1 weeks undergoing treatment at three-hour dialysis sessions for 3 days per week. The group had a mean age of 38.9 ± 9.98 years and consisted of 11 males. The peritoneal dialysis group consisted of 15 patients who were on treatment for a mean dialysis period of 208 ± 111.3 weeks. This group consisted of 11 males and 4 females with a mean age group of 36 ± 11.77 years. The age-, gender- and race-matched control group consisted of 12 healthy volunteers, 8 males, with a mean age of 35.9 ± 10.42 years. There was no statistically significant difference (p=0.7301) in age between the three groups. All patients and controls signed informed consent forms prior to taking part in the study and ethical clearance (S168/2006) was obtained from the local Ethics Committee in accordance with the Declaration of Helsinki.
Collection and processing of samples
Blood samples were collected from the patients and controls using a standard protocol for all subjects. Samples were collected in potassium EDTA, BD Vacutainer K2E 7.2mg, 4ml vacuum purple top tubes. This was done by venous puncturing of the antecubital vein in the controls and the peritoneal dialysis group. Blood from haemodialysis patients was collected at the initiation of dialysis, from the arterial line, and with a transmembrane pressure set at 0mmHg to prevent ultrafiltration influencing the levels of the substances to be analysed. Care was taken not to dilute the blood by the priming saline. Blood from peritoneal dialysis patients was collected before initiation of dialysis. All blood specimens, collected for the analysis of tryptophan and tryptophan metabolites, were collected after an overnight fast; immediately placed on ice before transportation for processing and storage. Samples for each of the study groups were collected during the same time frame thereby eliminating circadian differences between the different samples. The samples were centrifuged at 3000 revolutions per minute at 4 degrees Celsius for ten minutes. The resulting plasma was distributed and stored in aliquots of 0.5 ml each. The samples were stored at -700C until analysis.
Gas chromatography – mass spectrometry analysis
The three analytes, i.e. tryptophan, kynurenine and quinolinic acid, were quantified by a gas chromatography – mass spectrometry (GC-MS) method that was developed and validated in our laboratory. Briefly the GC-MS system consisted of a Hewlett-Packard HP5900 gas chromatograph coupled to an HP 5970 quadrupole massselective detector. A negative chemical ionisation source was used to detect the three analytes derivatized with pentafluro propionic anhydride (PFPA) and pentafluro propanol (PFP). Separation of analytes was achieved on a 30m DB-5-MS capillary GC column with a diameter of 250.0 μm and a film thickness 0.10 μm. The GC oven was temperature programmed to start at an initial temperature of 800C for 2 minutes, ramped at a rate of 200C to 2100C and held at 2100C for 1 minute. The final ramp was at a rate of 300C to 3000C and held at 3000C for 2 minutes. The chromatographic run time for a single analysis was 12.5 minutes using helium carrier gas with a flow rate of 2.5 ml/min. The mass spectrometer was operated in selective ion monitoring mode and the highest abundant ions for each analyte were used for quantification.
Results
Results of the volunteer control group, haemodialysis patient group and peritoneal dialysis patient group were compared by using Kruskal-Wallis one way analysis of variance testing and multiple comparison testing. The two patient groups were compared with each other using Mann-Whitney tests. Statistical inference for the analysis was performed using correction for non-parametric distribution of data with P values of less than 0.05 considered as statistically significant.
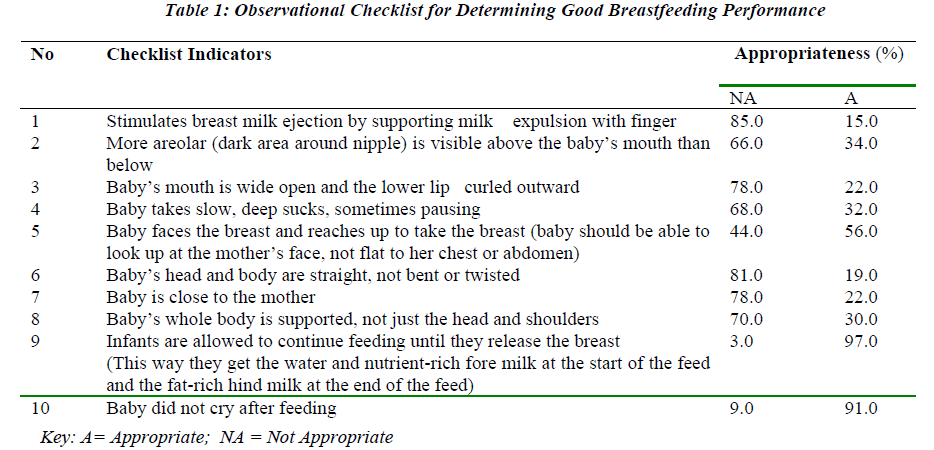
Table 1 shows the comparison between haemodialysis and peritoneal dialysis patients using Mann-Whitney test statistic (2 group comparison) for urea, albumin, haemoglobin and C-reactive protein (CRP) levels. This was performed using normal two-tail approximations. P-values of less than 0.05 were considered as statistically significant.
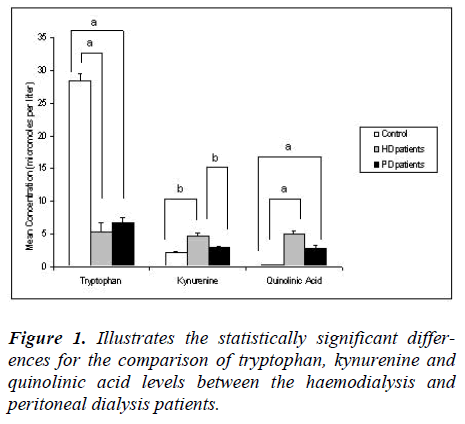
Tryptophan was significantly depleted (p<0.05) in the peritoneal dialysis patients (6.7 ± 3.2 μM) compared to the normal control group (28.4 ± 4.3 μM). There was no significant difference in tryptophan levels between the haemodialysis and peritoneal dialysis groups. The kynurenine levels in the peritoneal dialysis patients (2.9 ± 0.8 μM) were higher compared to the control group; however this did not reach statistical significance. In fact, the kynurenine levels in the peritoneal dialysis group were significantly lower (p<0.05) than in the haemodialysis group. Quinolinic acid levels were significantly higher (p<0.05) in the peritoneal dialysis group (2.8 ± 2.03 μM) than the control group. There was no significant difference between the quinolinic acid levels of the peritoneal dialysis and the haemodialysis group.
Discussion
Abnormalities of tryptophan metabolism such as depletion of tryptophan [23] and serotonin [26], as well as accumulation of kynurenine and quinolinic acid – metabolites of the kynurenine pathway of tryptophan metabolism, have previously been reported in ESRD by a small number of European studies. However, these studies, focussed mainly on ESRD patients on haemodialysis treatment.
This study simultaneously examined the levels of tryptophan, kynurenine and quinolinic acid in 15 haemodialysis and 15 peritoneal dialysis patients by the use of a newly developed and validated GC-MS technique. The patient groups were similar with respect to age, time they have been on renal replacement treatment, and with regard to uraemia as reflected by urea levels (urea: HD 27.7 ± 7.32 μM versus PD 25.2 ± 11.1 μM; p=0.38). Both patient groups had albumin levels below our laboratory reference range (HD 33.1 ± 2.8 versus PD 30.6 ± 5.4 g/L; p=0.23; reference range 34-48 g/L).
The results of our study compared relatively closely to a few other studies regarding the control and haemodialysis patient groups whereby different analytical methods were employed. A discussion on the most comparable studies is presented here. The possible reasons for the differences in patients’ tryptophan and metabolite levels will be explained in more detail in section 4.4.
Control group
The values for tryptophan, kynurenine and quinolinic acid levels found in our control group corresponded to that of the controls in some other studies whereby different methods were used. The tryptophan levels for the control group (28.4 ± 4.3 μM) were comparable to that of studies published by Pawlack et al. 2002 and by Myśliwiec et al. 2002 [27,28]. In a study on chronic renal failure patients, using reversed phase high performance liquid chromatography and fluorescence detection, plasma tryptophan levels of their control group of 16 healthy subjects were 31.2 ± 7.4 μM [27]. Myśliwiec et al. 2002, employing high performance liquid chromatography, found values of 25.15 μM for their normal controls [28]. The kynurenine levels for our control group (2.1 ± 0.6 μM) were comparable to those of Holmes et al. 1988 (2.26 ± 0.48 μM; high performance liquid chromatography) and that of Pawlak et al. 2002 (1.6 ± 0.9; high performance liquid chromatography) [27,29]. Quinolinic acid levels for our control group (0.3 ± 0.15 μM) compared well to the 0.26 ± 0.1 μM of Pawlak et al. 2002 [24] and 0.42 μM of Myśliwiec et al. 2002 [27,28]
Haemodialysis patients.
Tryptophan was significantly depleted (p<0.05) in our haemodialysis (5.3 ± 5.0 μM) compared to the control group (28.4 ± 4.3 μM). Although some previous studies indicated a tryptophan depletion in haemodialysis patients, their values were higher than our group, i.e., 17.3 ± 9.7 μM (Pawlak et al. 2002) and 15.2 μM (Myśliwiec et al. 2002 [27,28]. This represents a 5.4 fold decrease below control values in tryptophan in our study, compared to a 1.8 fold (Pawlak et al. 2002) and a 1.7 fold (Myśliwiec et al. 2002) decrease, with respect to their controls, in other studies [27,28].
Kynurenine levels were significantly higher (p<0.05) in our haemodialysis group (4.7 ± 1.9 μM), than in the control group (2.1 ± 0.6 μM). The kynurenine levels of the haemodialysis patients of the other studies were 2.7 ± 1.4 μM (Pawlak et al. 2002) and 3.99 ± 1.68 μM (Holmes et al. 1988) [27,29]. There was thus a 2.2 fold increase above normal in kynurenine for our study in comparison to 1.7 fold (Pawlak, Koda, Pawlak , Wolczynski, Buczko [24] and 1.8 fold of Holmes [29]. increases, relative to their controls, by other studies [27,29].
Quinolinic acid levels were elevated with a statistically significant difference (p<0.05) between haemodialysis (4.9 ± 2.03 μM) and the controls (0.3 ± 0.15 μM). The quinolinic acid levels of the haemodialysis patients of other studies were 7.9 ± 2.8 μM [27]; 6.75 μM [28] and 9.2 ± 5.3 μM [30]. In our study quinolinic acid showed a 16.3 fold increase above our control levels, which corresponds to the 15.3 fold increase above their control values [30], while other studies showed 30.4 fold [27] and 30.7 fold [28] increases above their control values.
Peritoneal dialysis patients
During peritoneal dialysis clearance of uraemic toxins and other unwanted substances occurs across the patient’s own peritoneal membranes as in contrast to haemodialysis where uraemic toxins filter across the membranes of the artificial kidney which in our patient group consisted of hollow fibre regenerated cellulose membranes. The results our peritoneal dialysis patients were similar to that found in haemodialysis patients of other groups, i.e., a 4.2 fold decrease in tryptophan, a 1.4 fold increase in kynurenine and a 9.3 fold increase in quinolinic acid.
Nutritional status in ESRD
The decreases of nutritional substances in the blood of ESRD patients are probably multifactorial. Renal failure patients are known to have alterations to their protein intake, both as a result of a decrease in appetite and as a result of certain dietary restrictions imposed by their therapy [31]. Albumin, although not a good indicator of protein intake in renal failure patients, remains a fair indicator of nutritional status. In this study albumin was found to be below normal in 53.3 % and 67.7% of the haemodialysis and peritoneal dialysis groups, respectively. There was no significant difference between the albumin levels of the haemodialysis and peritoneal dialysis groups (p=0.2339), neither could any significant difference be seen between the tryptophan levels of the two patient groups investigated. Dietary causes can thus, with a fair amount of certainty, be assumed to be a contributor to the albumin, as well as to the tryptophan depletion, seen in this study. However, there is a real possibility that proinflammatory activity could have contributed to the tryptophan depletion, as well as to the excessive production of the metabolites of the kynurenine pathway. The reason for this is that an increase in the pro-inflammatory activity causes an induction of the enzyme IDO that is in turn responsible for the conversion of tryptophan to kynurenine [22,32]. There are many factors that could potentially contribute to increased pro-inflammatory activity in endstage renal failure patients – especially those on renal replacement therapies. It is, for instance, acknowledged that the haemodialysis procedure itself, partially as a result of the use of bio-incompatible membranes, and the high endotoxin levels could result in immune activation and release of pro-inflammatory cytokines [33,34]. Proinflammatory conditions may also contribute to a lowering of plasma albumin by suppressing albumin synthesis [14] and leading to the movement of albumin from the blood into the interstitial compartment. Sufficient evidence exists to believe that end-stage renal failure patients on renal replacement therapies indeed have enhanced immune activation [16]. For instance, increased neopterin levels were found in long-term haemodialysis patients; serum C-reactive-protein (CRP) and pro-inflammatory cytokine levels were reported to be higher in haemodialysis patients and neopterin was found to be high in chronic renal failure patients [35,36,37].
In this study the possibility that immune activity, known to stimulate a shift in the tryptophan metabolic pathways, could have been present was examined by looking at Creactive protein (CRP) levels in the patient groups. The normal reference range for the CRP laboratory test is 0 to 10 mg/L. Seven haemodialysis and seven peritoneal dialysis patients had CRP levels above 10 mg/L. This needs to be further investigated. It has to be stressed that previous studies were done in first world countries and that the majority of the patients in our study were from a tertiary academic hospital and thus from the previously disadvantaged populations in South Africa. Malnutrition and infections could therefore have been more prevalent. Both tryptophan depletion and kynurenine metabolite accumulation can have negative consequences for patients.
Limitations
This study was confined to one dialysis centre. These results should be confirmed by a multi-centre study where not only tryptophan and its metabolites are measured, but where the nutritional and pro-inflammatory status of all individual ESRD patients are assessed beyond albumin and CRP levels. Although the sample size of this study was relatively small, the typical tryptophan metabolic profile could be seen in the patient groups. This should however be warranted with future studies involving larger study populations.
Conclusion
The results of this study supported that of the small number of other studies that showed depletion of tryptophan and accumulation of kynurenine and quinolinic acid in the blood of haemodialysis patients. Tryptophan depletion in the present study was however much more pronounced. The levels in ESRD were similar in haemodialysis and peritoneal dialysis, except for lower kynurenine levels in the peritoneal dialysis group. Malnutrition and a higher infection rate on the African Continent could have contributed to the magnitude of tryptophan depletion, and perhaps a greater shift towards kynurenine and quinolinic acid as seen in this study.
References
- Tripepi G, Mallamaci F, Zoccali C. Inflammation markers, adhesion molecules, and all-cause and cardiovascular mortality in patients with ESRD: Searching for the best risk marker by multivariate modeling. J Am Soc Nephrol. 2005; 16 (1): 83-88.
- Yavuz A, Tetta C, Ersoy FF, D’intini V, ratanarat R, De Cal M, Bonello M, Bordoni V, Salvatori G, Andrikos E, Uakupoglu G, Levin NW, Ronco C. Uremic Toxins: A new focus on an old subject. Semin Dial. 2005; 18 (3): 203-211.
- Stenvinkel P, Heimbürger O, Lindholm B, Kaysen GA, Bergström J. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome). Nephrol Dial Transplant. 2000; 15: 953-960.
- Bergström J. Why are dialysis patients malnourished? Am J Kidney Dis. 1995; 26 (1): 229-241.
- Abdullah MS, Wild G, Jacob V, Milford-Ward A, Ryad R, Zanaty M, Ali MH, el Nahas A. Cytokines and the malnutrition of chronic renal failure. Miner Electrolyte Metab. 1997; 23 (3-6): 237-242.
- Herselman M, Moosa MR, Kotze TJ, Kritzinger M, Wuister S, Mostert D. Protein-energy malnutrition as a risk factor for increased morbidity in long-term hemodialysis patients. J Ren Nutr. 2000; 10 (1): 7-15.
- Marckmann P. Nutritional status of patients on hemodialysis and peritoneal dialysis. Clin Nephrol. 1988; 28: 75-78.
- Bergström J, Lindholm B. Nutrition and adequacy of dialysis. How do hemodialysis and CAPD compare? Kidney Int. 1993; 34: S39.
- Cianciaruso B, Brunori G, Kopple JD et al. Crosssectional comparison of malnutrition in continuous ambulatory dialysis and hemodialysis patients. Am J Kidney Dis. 1995; 26: 475-483.
- Qureshi AR, Alvestrand A, Danielsson A, Divino-Filho JC, Gutierrez A, Lindholm B et al. Factors influencing malnutrition in hemodialysis patients. A cross-sectional study. Kidney Int. 1998; 53: 773-782.
- Stenvinkel P, Heimbürger O, Paultre F, et al. Strong associations between malnutrition, inflammation and atherosclerosis in chronic renal failure. Kidney Int. 1999; 55: 1899-1911.
- Hakim RM, Levin N. Malnutrition in hemodialysis patients. Am J Kidney Dis. 1993; 21: 125-137.
- Guarnieri G, Toigo G, Fiotti N, Ciocchi B, Situlin R, Giansante C, Vasile A, Carraro M, Faccini L, Biolo G. Mechanisms of malnutrition in uremia. Kidney Int Suppl. 1997; 62: 41-44.
- Kalantar-Zadeh K, Ikizler A, Block G, Avram MM, Kopple JD. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis. 2003; 42 (5): 864-881.
- Honda H, Qureshi AR, Heimbürger O, Barany P, Wang K, Pecoits-Filho R, Stenvinkel P. Serum albumin, Creactive protein, interleukin 6 and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis. 2006; 47 (1): 139-148.
- Zoccali C, Tripepi G, Mallamaci F. Dissecting Inflammation in ESRD: Do Cytokines and C-Reactive Protein Have a Complementary Prognostic Value for Mortality in Dialysis Patients. J Am Soc Nephrol. 2006; 17: 169- 173.
- Russo S, Kema IP, Fokkema MR, Boon JC, Willemse PHB, de Vries EGE, den Boer JA, Korf J. Tryptophan as a link between psychopathology and somatic states. Psychosom Med. 2003; 65 (4): 665-671.
- Peters JC. Tryptophan nutrition and metabolism: an overview. Adv Experim Med Biol. 1991; 294: 345-358.
- Eynard N, Flachaire E, Lestra C, Broyer M, Zaidan R, Claustrat B, Quincy C. Platelet Serotonin content and free and total plasma tryptophan in healthy volunteers during 24 hours. Clin Chem. 1993; 39: 2337-2340.
- Myint A, Kim JK, Verkerk R, Scharp S, Steinbusch H and Leonard B. Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord. 2007; 98 (1-2): 143-151.
- Li JS, Han Q, Fang J, Rizzi M, James AA, Li J. Biochemical mechanisms leading to tryptophan 2,3- dioxygenase activation. Arch Insect Biochem Physiol. 2007; 64 (2): 74-87.
- Thomas SR, Stocker R. Redox reactions related to indoleamine 2,3-dioxygenase and tryptophan metabolism along the kynurenine pathway. Redox Rep. 1999; 4 (5): 199-220.
- Pawlak D, Pawlak K, Malyszko J, Mysliwiec M and Buczko W. Accumulation of toxic products degeneration of kynurenine in hemodialyzed patients. Int Urol Nephrol. 2001; 33: 399-404.
- Pawlak D, Koda M, Pawlak S, Wolczynski S and Buczko W. Contribution of quinolinic acid in the development of anemia in renal insufficiency. Am J Physiol Renal Physiol. 2003; 284: 693-700.
- Mellor AL, Munn DH. Tryptophan catabolism and Tcell tolerance: immunosuppression by starvation. Immunol Today. 1999; 20 (10):469-473.
- Steyn ME, Viljoen M, Ubbink JB, van Rensburg BW, Reinach SG. Whole blood serotonin levels in chronic renal failure. Life Sci. 1992; 51 (5): 359-366.
- Pawlak D, Tankiewicz A, Mysliwiec P, Buczko W. Tryptophan metabolism via the kynurenine pathway in experimental chronic renal failure. Nephron. 2002; 90 (3): 328-325.
- Myśliwiec P, Pawlak D, Myśliwiec M, Buczko W. Endogenous neurotoxin-quinolinic acid is increased in renal allograft recipients. Transplant Proc. 2002; 34: 598-600.
- Holmes EW. Determination of serum kynurenine and hepatic tryptophan dioxygenase activity by highperformance liquid chromatography. Anal Biochem. 1988; 172 (2): 518-525.
- Niwa T, Yoshizumi H, Emoto Y, Miyazaki T, Hashimoto N, Takeda N, Tatmatsu A, Maeda K. Accumulation of quinolinic acid in uremic serum and its removal by hemodialysis. Clin Chem. 1991; 37 (2): 159-161.
- Gutch CF, Stoner MH. Review of haemodialysis for nurses and dialysis personnel. Mosby. 1983; 4: 128- 136.
- Schefold JC, Zeden J, Fotopoulou C, von Haehling S, Pschowski R, Hasper D, Volk H, Schuett C, reinke P. Increased indoleamine 2,3-dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: a possible link between chronic inflammation and uraemic symptoms. Nephrol Dial Transplant 2009; 24: 1901-1908. 33.
- Lim VS, Kopple JD. Protein metabolism in patients with chronic renal failure: Role of uremia and dialysis. Kidney Int. 2000; 58: 1-10.
- Yao Q, Axelsson J, Heimburger O, Stenviinkel P, Lindholm B. Systemic inflammation in dialysis patients with end-stage renal disease: causes and consequences. Minerva Urol Nefrol. 2004; 56: 237-248.
- Fuchs D, Hausen A, Reibnegger G, Werner G, von Dittrich ER, Wachter H. Neopterin levels in long-term hemodialysis. Clin Nephrol. 1988; 30(4): 220-224.
- Borazan A, Ustun H, Ustundag Y, Aydemir S, Bayraktaroglu T, Sert M, Yilmaz A. The effects of peritoneal dialysis and hemodialysis on serum tumor necrosis factor- alpha, interleukin-6, interleukin 10 and C-reactiveprotein levels. Mediators Inflamm. 2004; 13 (3): 201- 204.
- Viljoen M, May M, Coetzee IH, Van Rensburg BWJ. Immunological significance of neopterin in chronic renal failure patients. Med Sci Res. 1994; 22: 59-60.