Research Article - Biomedical Research (2017) Volume 28, Issue 9
Triptolide protects bone against destruction by targeting RANKL-mediated ERK/AKT signalling pathway in the collagen-induced rheumatoid arthritis
Yan Gong1, Xiaopeng Huang1*, Di Wang1, Ming Li2 and Zhongyuan Liu11Department of Orthopaedics, Shanghai Yang Si hospital, Shanghai, China
2College of Basic Medicine, Beijing Medical College, Beijing, China
Accepted date: February 07, 2017
Abstract
Rheumatoid Arthritis (RA) is a chronic autoimmune disease characterized by the inflammatory synovitis and the destructive cartilage and bone. Triptolide is a biologically active component purified from the Chinese herbal plant Tripterygium wilfordii Hook F (TWHF), which has presented benefits for the treatment of RA. In the current study, we investigated therapeutic effects of Triptolide in collageninduced rheumatoid arthritis rat model. Administration of Triptolide (30 μg/kg) was used to treat Collagen-Induced Arthritis (CIA) rat model by the subcutaneous injection of bovine type II collagen. Our data demonstrated the triptolide treatment significantly reduced inflammatory cytokines including IL-1β, TNF-α, IL-17 and IL-8 in serum in experimental rats. Results demonstrated that triptolide treatment reduced the expression of angiogenic activators Vascular Endothelial Growth Factor (VEGF), Toll-like receptor 2 (Tie2), Angiogenin-1 (Ang-1) and Angiogenin-2 (Ang-2) in synovial cells in experimental rats. Potential mechanism analyses triptolide treatment inhibited expression levels of receptor activator of NF-κB (RANK) ligand (RANKL), which further suppressed RANKL-induced expression and phosphorylation of ERK and AKT at protein levels. Histological analysis showed that triptolide treatment decreased immune cell infiltration and suppressed bone destruction that contributed to attenuation of arthritis severity in CIA rats. In conclusion, these results indicate that triptolide treatment not only inhibits inflammatory cytokines, but also possess anti-angiogenic effect in RA both in vivo and in vitro experiments through down-regulation of RANKL-mediated ERK/AKT signalling pathway, which contributes to understand molecular mechanism mediated by triptolide in the progression of RA.
Keywords
Rheumatoid arthritis, Triptolide, Inflammation, RANKL, ERK/AKT, Bone destruction
Introduction
Rheumatoid Arthritis (RA) is one of complicated arthritis diseases caused by various factors, such as environmental, drug stimulation, and genetic factors [1]. RA presents an intractable autoimmune disease that is characterized overactive immune system and synovial inflammation in clinical patients which further leads to cartilage and synovial injury [2,3]. Reports have showed that RA will ultimately result in irreversible joint destruction and severe disability due to non-effective treatments [4,5]. Notably, inflammatory cytokines and vascularisation markers are important pathological factors in progression of rheumatoid arthritis, which may be significant impact not only on chronic inflammation but also on acute inflammatory processes for RA therapy [6,7].
Currently, a large numbers of therapeutic strategies for RA patients in clinic including non-steroidal anti-inflammatory drugs, disease modifying antirheumatic drugs, steroid and biological response modifiers, which could inhibit inflammation and angiogenic activators to relieve RA progression and further preventing the subsequent damage of joint [8,9]. Evidences have suggested that Chinese herbal plant triptolide plays important role in the treatment of RA by regulation of inflammatory cytokines through regulation of different signal pathways [10,11]. Yao et al. have suggested that triptolide could inhibit expression of COX-2 and iNOS in human rheumatoid arthritis synovial fibroblasts that contributes to repair of articular cartilage by suppressing the activity of NF-kappa B [12]. In addition, the important role of MEK/ERK MAP kinase pathway has been identified to confirm the potential proinflammatory mechanisms in a mouse model of rheumatoid arthritis [13]. However, triptolide-mediated RANKL-mediated ERK/AKT signal pathway has not been investigated in collagen-induced rheumatoid arthritis.
Given the potential application of triptolide for the treatment of autoimmune and inflammatory diseases, we investigated the inflammation inhibiting function and potential molecular mechanism in synovial cells in CIA-induced RA rat. Our analyses researched benefits of joint swelling, periosteal new bone formation, articular bone erosion, and osteopenia after treatment with triptolide compared to dexamethasone and NaCl groups. Findings suggest that triptolide may be a potential agent for the treatment of RA.
Materials and Methods
Ethic statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals. All experimental protocols and animals were performed in accordance with National Institutes of Health and approved by Committee on the Ethics of Animal Experiments Defence Research. All surgery and euthanasia were made to minimize suffering.
Animal study
Six-eight weeks old male SD rat were purchased from commercial company (Shanghai SLAC laboratory animals Co. Ltd). All rats were identified by ear punching and used to establish CIA-induced (100 mg/kg) RA rat model according to previous report [14]. The rats were divided into three groups and given a normal schedule and free access to a standard diet and water. On day 7 after model establishment, rats were received treatment with triptolide (30 μg/kg, Sigma Aldrich Co., St. Louis, MO, USA), DEX (10 mg/kg, Sigma Aldrich Co., St. Louis, MO, USA) and the same volume of NaCl by subcutaneous injection. Treatments were continued seven times at an interval of 4 day. All rats were sacrificed on day 32 for histological analysis. Clinical RA scores were evaluated using a scale of 0-10 as previously described [15].
Cells and reagents
Synovial cells were isolated from experimental rats and cultured in DMEM medium (Gibco, CA, USA) supplemented with 10% Fetal Bovine Serum (FBS) (Invitrogen, CA, USA). Cells were cultured in a 37°C humidified atmosphere of 5% CO2.
Transfection of small interference RNA (Si-RNA)
All siRNAs were synthesized by Invitrogen (Shanghai, China) including Si-RNA-RANKL (Si-RANKL) or Si-RNA-vector (Si-vector). Synovial cells (1 × 106) were transfected with 100 pmol of Si-RANKL for silencing RANKL (Applied Biosystems) with Si-RNA-vector as control (Applied Biosystems) by using a Cell Line Nucleofector kit L (Lonza).
Endogenous overexpression of RANKL
Synovial cells were cultured until 85% confluence and the media was then removed from cultural system. Synovial cells were then transfected by pedue6.4-RANKL using lipfectamine 2000 (Sigma-Aldrich). Stable RANKL-overexpression synovial cells were selected by GS screening system [16].
ELISA
For protein detection assay, ELISA kits (R&D, Bio-Techne China Co. Ltd) were used to determine mouse IL-17 (DY421, Bio-Techne China Co. Ltd), TNF-α (P01375, Bio-Techne China Co. Ltd), IL-8 (D8000C, Bio-Techne China Co. Ltd) and IL-1β (201-LB, Bio-Techne China Co. Ltd) serum levels. The procedures were conducted according to the manufacturer’s instructions. The final results were recorded at 450 nm on an ELISA plate reader.
Western blotting
Synovial cells were isolated from experimental rats and homogenized in lysate buffer containing protease-inhibitor and were centrifuged at 8000 rpm/min at 4°C for 10 min. The supernatant of mixture were used for analysis of purpose protein. The purpose protein expression levels were analysed via SDS assays. For western blotting, primary antibodies were added after blocking (5% skimmed milk) for 1 h at 37°C and then incubating with HRP-connected second antibodies 24 h at 4 °C. The results were visualized by using chemi-luminescence detection system.
Immunohistochemistry
Synovial tissues were from experimental rat were fixed by using formaldehyde (10%) followed with embed in paraffin and fabricated to tissue sections. Antigen retrieval was performed in tissues sections and the sections were incubated with primary antibodies targeting of CD31 (1:200, Vector Laboratories (Burlingame, CA). Then, appropriate poly-HRP anti-goat IgG secondary antibodies were applied for specimens and specimens were visualized. A Ventana Benchmark automated staining system was used for observation lymphocytes infiltration.
Histopathologic analysis
The RA rats were sacrificed under pentobarbital anesthesia on day 32 and the raw joints were separated and fixed in 10% formalin. The joints were subsequently decalcified and embedded in paraffin. The raw joints from experimental rats were stained with Haematoxylin and eosin (H and E). Cartilage and bone destruction and the hyperplasia of synovial in CIA rats were analysed by H and E staining. The severity of arthritis in the joints was scored on a scale of 0-10 descripted in previous study [17].
Statistical analysis
All date were expressed as mean and SD of triplicate dependent experiments and analysed by using student t tests or one-way ANOVA (Tukey HSD test). All data were analysed using SPSS Statistics 19.0 and Graphpad Prism version 5.0. *P<0.05 and **P<0.01 were considered statistical differences.
Results
Triptolide treatment inhibits serum levels of inflammatory cytokines in CIA-induced RA rat
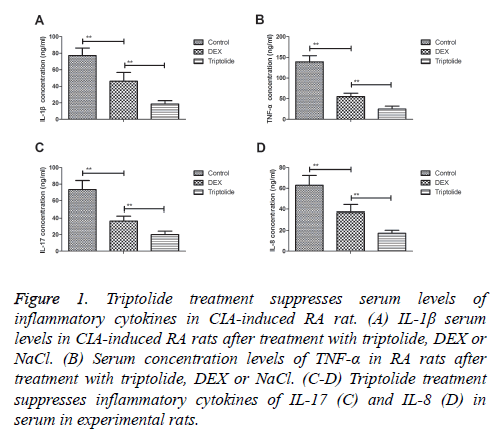
Serum levels of inflammatory cytokines were investigated in CIA-induced RA rat. We observed triptolide treatment inhibited IL-1β serum levels were decreased compared to DEX and NaCl groups (Figure 1A). Serum concentration levels of TNF-α were down-regulated by triptolide in CIA-induced RA rat (Figure 1B). Inflammatory cytokines of IL-17 and IL-8 were also significantly decreased by triptolide compared to DEX- or Nacl-treated CIA rat (Figures 1C and 1D). These data suggest that triptolide treatment significantly inhibits serum levels of inflammatory cytokines in CIA-induced RA rat.
Figure 1: Triptolide treatment suppresses serum levels of inflammatory cytokines in CIA-induced RA rat. (A) IL-1β serum levels in CIA-induced RA rats after treatment with triptolide, DEX or NaCl. (B) Serum concentration levels of TNF-α in RA rats after treatment with triptolide, DEX or NaCl. (C-D) Triptolide treatment suppresses inflammatory cytokines of IL-17 (C) and IL-8 (D) in serum in experimental rats.
Triptolide treatment suppresses angiogenic activators in synovial cells in CIA-induced RA rat
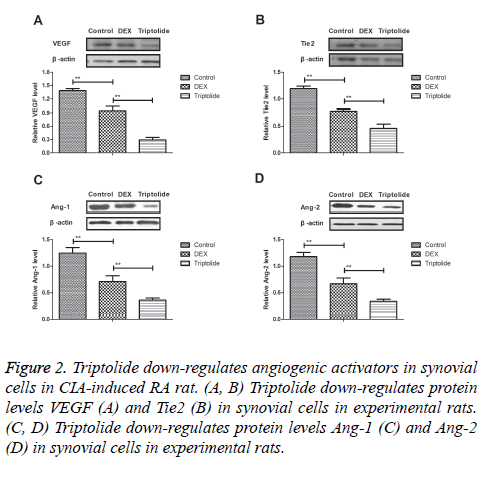
Angiogenic activators play essential role for the progression of CIA-induced RA rat. Results demonstrated that triptolide treatment inhibited protein levels VEGF and Tie2 were down-regulated in synovial cells in experimental rats (Figures 2A and 2B). Expression levels of Ang-1 and Ang-2 were also markedly decreased by 28-day triptolide treatment in CIA-induced RA rat (Figures 2C and 2D). Collectively, results indicate triptolide treatment significantly suppresses angiogenic activators in synovial cells in CIA-induced RA rat.
Figure 2: Triptolide down-regulates angiogenic activators in synovial cells in CIA-induced RA rat. (A, B) Triptolide down-regulates protein levels VEGF (A) and Tie2 (B) in synovial cells in experimental rats. (C, D) Triptolide down-regulates protein levels Ang-1 (C) and Ang-2 (D) in synovial cells in experimental rats.
Triptolide improves severity of arthritis in synovial cells in CIA-induced RA rat
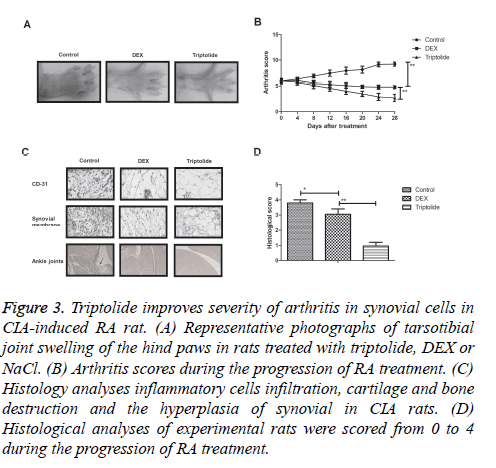
To further analyse the beneficial effects of triptolide treatment on the progression of RA, CIA rat model was used to study the in vivo efficacy. Results showed that triptolide treatment significantly alleviated the severity of arthritis determined by the arthritic scores of rats (Figures 3A and 3B). Histological analyses showed that triptolide treatment inhibited the inflammatory cells infiltration, cartilage and bone destruction and the hyperplasia of synovial in CIA rats (Figures 3C and 3D). Taken together, these results suggest that triptolide treatment improves histopathological changes for CIA rats in vivo.
Figure 3: Triptolide improves severity of arthritis in synovial cells in CIA-induced RA rat. (A) Representative photographs of tarsotibial joint swelling of the hind paws in rats treated with triptolide, DEX or NaCl. (B) Arthritis scores during the progression of RA treatment. (C) Histology analyses inflammatory cells infiltration, cartilage and bone destruction and the hyperplasia of synovial in CIA rats. (D) Histological analyses of experimental rats were scored from 0 to 4 during the progression of RA treatment.
Triptolide suppresses RANKL-mediated ERK/AKT signal pathway in synovial cells in CIA-induced RA rat
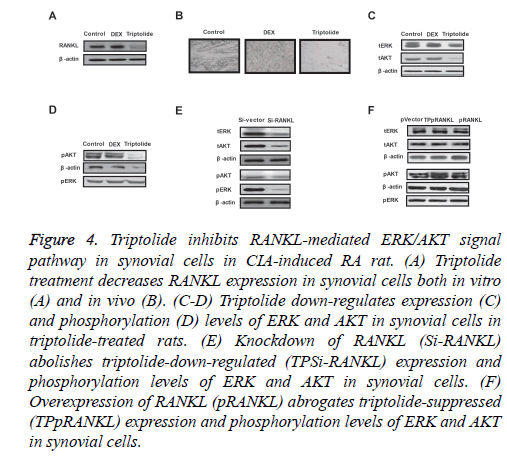
In order to analyse triptolide-mediated benefits for articular cartilage repair, we investigated RANKL-mediated ERK/AKT signal pathway in synovial cells in CIA-induced RA rat. Our results demonstrated that triptolide treatment decreased RANKL expression in synovial cells both in vitro and in vivo (Figures 4A and 4B). Western blot showed that expression and phosphorylation levels of ERK and AKT were decreased in synovial cells in triptolide-treated rats (Figures 4C and 4D). Results showed that knockdown of RANKL (Si-RANKL) suppressed expression and phosphorylation levels of ERK and AKT in synovial cells (Figure 4E). Overexpression of RANKL (pRANKL) abrogated triptolide-suppressed (TPpRANKL) expression and phosphorylation levels of ERK and AKT in synovial cells (Figure 4F). These data suggest that triptolide can suppress RANKL-mediated ERK/AKT signal pathway in synovial cells in CIA-induced RA rat.
Figure 4: Triptolide inhibits RANKL-mediated ERK/AKT signal pathway in synovial cells in CIA-induced RA rat. (A) Triptolide treatment decreases RANKL expression in synovial cells both in vitro (A) and in vivo (B). (C-D) Triptolide down-regulates expression (C) and phosphorylation (D) levels of ERK and AKT in synovial cells in triptolide-treated rats. (E) Knockdown of RANKL (Si-RANKL) abolishes triptolide-down-regulated (TPSi-RANKL) expression and phosphorylation levels of ERK and AKT in synovial cells. (F) Overexpression of RANKL (pRANKL) abrogates triptolide-suppressed (TPpRANKL) expression and phosphorylation levels of ERK and AKT in synovial cells.
Discussion
Reports have suggested that inflammatory cytokines and angiogenic activators play essential role in the progression of RA, which could lead to synovial injuries and joint diseases by targeting of synovial cells [18-20]. Evidences also indicated that administration of triptolide is beneficial for inhibition of inflammatory response and presents anti-angiogenic effects in RA by targeting angiogenic cascade and TREM-1 signal pathway [21,22]. Notably, researches have indicated that ERK/AKT signal pathway involves in the progression of autoimmune-mediated inflammatory responses and proliferation and aberrant survival of activated immune cells, macrophages, monocytes, dendritic cells and synovial fibroblasts in synovium fluid in the development of RA [23,24]. In this study, we explored therapeutic effects of Triptolide for CIA-induced RA as well as potential molecular mechanism in synovial cells in experimental rats. Our results indicate that triptolide treatment not only inhibits inflammatory cytokines, but also suppresses angiogenic activators in synovial cells in CIA-induced RA rat. Findings have suggested that triptolide treatment improves severity of arthritis by down-regulation of RANKL-mediated ERK/AKT signal pathway in synovial cells in CIA-induced RA rat.
Currently, inflammatory cytokines increase the cartilage and bone destruction and the hyperplasia of synovial in CIA rats [25]. IL-17 might play a crucial role in the pathogenesis of osteoarthritis and is closely related to joint pain and damages [26]. Treatment with the first TNF inhibitor in rheumatoid arthritis patients improves quality of life especially in young patients with better baseline functional status by inhibition of TNF-α content in synovial cells [27]. Meta-analysis has showed that IL-1 and IL-8 serum levels were up-regulated in patients with RA and down-regulation of IL-1 and IL-8 levels contribute to decreasing of inflammatory infiltration for synovial cells [28-31]. Our results demonstrated that triptolide treatment down-regulates inflammatory cytokines of TNF-α, IL-17, IL-1 and IL-8 in serum in CIA rats.
Up-regulation of angiogenic activators has been found in patients with RA. Malemud et al. have showed that VEGF involves in rheumatoid arthritis and have also been implicated in aberrant synoviocyte proliferation and apoptosis resistance in adult RA [32]. Saber et al. indicate that Tie2 could induce angiogenesis and invasion through the Tie2 signalling pathway in rheumatoid arthritis, which may be a potential target for understanding the mechanisms involved in the pathogenesis of RA [33]. Strunk et al. have showed that angiogenin can be regarded as potential target molecular and anti-inflammatory treatment could decrease in patients with rheumatoid arthritis [34]. Our investigations have showed that triptolide treatment down-regulates angiogenic activators of VEGF, Tie2, Ang-1 and Ang-2 in serum in CIA rats, which further decreases infiltrating inflammatory markers on synovial cells.
Previous studies have indicated that blocking ERK-1/2 reduces tumor necrosis factor alpha-induced interleukin-18 bioactivity in rheumatoid arthritis synovial fibroblasts [35], and AKT activity is stimulated in rheumatoid arthritis and normal fibroblast-like synoviocytes in response to tumor necrosis factor alpha [36]. In addition, inhibition of the inflammatory responses in rheumatoid arthritis fibroblast-like synoviocytes through regulating the Raf/ERK and PTEN/AKT signals contribute to synovial repair [37]. Furthermore, RANKL is over-expressed in the synovium of RA and serum RANKL levels associate with anti-citrullinated protein antibodies in early untreated rheumatoid arthritis and are modulated following methotrexate [38,39]. In this study, our data indicate that triptolide treatment decreases severity of arthritis through observing histopathological changes via down-regulation of RANKL-mediated ERK/AKT signal pathway in CIA-induced RA rat.
In conclusion, findings in this study indicate that triptolide treatment may possess anti-inflammation and anti-angiogenic effects as well as inflammatory cells infiltration, cartilage and bone destruction and the hyperplasia of synovial in CIA rats both in vivo and in vitro assay via down-regulation of RANKL-mediated ERK/AKT signal pathway. These observations provide a promising insight into the therapeutic value of triptolide in RA pathogenesis, which helps us to understand the potential molecular mechanism in the progression of RA.
Conflict of Interest
All authors declare no conflicts of interest.
References
- Kochi Y, Suzuki A, Yamamoto K. Genetic basis of rheumatoid arthritis: a current review. Biochem Biophys Res Commun 2014; 452: 254-262.
- Tahmasebi MN, Bashti K, Sobhan M, Ghorbani G. Bilateral synovial knee chondromatosis in a patient with rheumatoid arthritis: case-report and literature review. Arch Bone Jt Surg 2014; 2: 260-264.
- Besada E. Potential patient benefit of a subcutaneous formulation of tocilizumab for the treatment of rheumatoid arthritis: a critical review. Patient Preference Adherence 2014; 8: 1051-1059.
- Nasir SH, Troynikov O, Massy-Westropp N. Therapy gloves for patients with rheumatoid arthritis: a review. Therap Adv Musculoskel Dis 2014; 6: 226-237.
- Matcham F, Scott IC, Rayner L, Hotopf M, Kingsley GH. The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: a systematic review and meta-analysis. Semin Arthritis Rheum 2014; 44: 123-130.
- Vordenbaumen S, Sewerin P, Logters T. Inflammation and vascularisation markers of arthroscopically-guided finger joint synovial biospies reflect global disease activity in rheumatoid arthritis. Clin Exp Rheumatol 2014; 32: 117-120.
- Ternant D, Ducourau E, Perdriger A. Relationship between inflammation and infliximab pharmacokinetics in rheumatoid arthritis. Br J Clin Pharmacol 2014; 78: 118-128.
- Smolen JS, Aletaha D, Gruben D, Zwillich SH, Krishnaswami S, Mebus C. Remission rates with tofacitinib treatment in rheumatoid arthritis: a comparison of various remission criteria. Arthritis Rheumatol 2016.
- Schafer VS, Schmidt WA, Backhaus M, Hartung W. Arthritis of the knee joint in rheumatoid arthritis - evaluation of treatment response by ultrasound in daily clinical practice. Open Rheumatol J 2016; 10: 81-87.
- Peng AP, Wang XY, Zhuang JH. Triptolide inhibits Th17 cell differentiation via regulating cyclooxygenase-2/ prostaglandin E2 axis in synovial fibroblasts from rheumatoid arthritis. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J Chin Materia Medica 2014; 39: 536-539.
- Peng A, Huang X, Liu R, Wang X, Zhuang J. Triptolide inhibits the inflammatory response of monocytes from rheumatoid arthritis patients by regulating miR-155. Chin J Cell Mol Immunol 2014; 30: 635-638.
- Yao HP, Shao XT, Sun WJ, Shao CS, Zhang LH. Enhancement by FK506 of triptolide-induced inhibition of expression of COX-2 and iNOS in human rheumatoid arthritis synovial fibroblasts. Bao Acta Biochimica Biophysica Sinica 2003; 35: 277-282.
- Thiel MJ, Schaefer CJ, Lesch ME. Central role of the MEK/ERK MAP kinase pathway in a mouse model of rheumatoid arthritis: potential proinflammatory mechanisms. Arthritis Rheumat 2007; 56: 3347-3357.
- Marcińska K, Szczepanik M. Mechanisms involved in the regulation of immune response in animal model of rheumatoid arthritis in mice (CIA). Postepy Hig Med Dosw (Online) 2010; 64: 372-385.
- Zalavras CG, Lieberman JR. Osteonecrosis of the femoral head: evaluation and treatment. J Am Acad Orthop Surg 2014; 22: 455-464.
- Renshaw A, Elsheikh TM. A validation study of the Focalpoint GS imaging system for gynecologic cytology screening. Cancer Cytopathol 2013; 121: 737-738.
- Conceicao CS, Neto MG, Neto AC, Mendes SM, Baptista AF, Sa KN. Analysis of the psychometric properties of the American Orthopaedic Foot and Ankle Society Score (AOFAS) in rheumatoid arthritis patients: application of the Rasch model. Rev Bras Reumatol Engl Ed 2016; 56: 8-13.
- Hodge JA, Kawabata TT, Krishnaswami S. The mechanism of action of tofacitinib - an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol 2016.
- van der Goes MC, Jacobs JW, Bijlsma JW. Rediscovering the therapeutic use of glucocorticoids in rheumatoid arthritis. Curr Opin Rheumatol 2016; 28: 289-296.
- Gu E, Lu J, Xing D. Rs7574865 polymorphism in signal transducers and activators of transcription 4 gene and rheumatoid arthritis: an updated meta-analysis of 28 case-control comparisons. Int J Rheum Dis 2015; 18: 3-16.
- Fan D, He X, Bian Y. Triptolide modulates TREM-1 signal pathway to inhibit the inflammatory response in rheumatoid arthritis. Int J Mol Sci 2016; 17: 498.
- Kong X, Zhang Y, Liu C, Guo W, Li X. Anti-angiogenic effect of triptolide in rheumatoid arthritis by targeting angiogenic cascade. PLoS One 2013; 8: e77513.
- Malemud CJ. The PI3K/Akt/PTEN/mTOR pathway: a fruitful target for inducing cell death in rheumatoid arthritis? Future Med Chem 2015; 7: 1137-1147.
- Ohori M. ERK inhibitors as a potential new therapy for rheumatoid arthritis. Drug News Perspect 2008; 21: 245-250.
- Krabbe S, Ammitzboll-Danielsen M, Ostergaard M, Giard MC, Terslev L. Sensitivity and specificity of optical spectral transmission imaging in detecting joint inflammation in rheumatoid arthritis. Ann Rheum Dis 2016; 75: 632-633.
- Liu Y, Peng H, Meng Z, Wei M. Correlation of IL-17 Level in Synovia and Severity of Knee Osteoarthritis. Med Sci Monitor Int Med J Exp Clin Res 2015; 21: 1732-1736.
- Boubouchairopoulou N, Flouri I, Drosos AA, Boki K, Settas L. Treatment with the first TNF inhibitor in rheumatoid arthritis patients in the Hellenic Registry of Biologic Therapies improves quality of life especially in young patients with better baseline functional status. Clin Exp Rheumatol 2016; 34: 999-1005.
- Lee YH, Bae SC. Associations between interleukin-1 and IL-1 receptor antagonist polymorphisms and susceptibility to rheumatoid arthritis: A meta-analysis. Cell Mol Biol (Noisy-le-grand) 2015; 61: 105-111.
- Ruscitti P, Cipriani P, Cantarini L, Liakouli V, Vitale A. Efficacy of inhibition of IL-1 in patients with rheumatoid arthritis and type 2 diabetes mellitus: two case reports and review of the literature. J Med Case Rep 2015; 9: 123.
- Luo XJ, Mo XR, Zhou LL. The effect of Hsp72 on IL-6, IL-8 expression and activation of NF-kappaB in synoviocytes of rheumatoid arthritis. Chin J Appl Physiol 2012; 28: 336-339.
- Lo SF, Huang CM, Lin HC, Chen WC, Tsai CH, Tsai FJ. Cytokine (IL-6) and chemokine (IL-8) gene polymorphisms among rheumatoid arthritis patients in Taiwan. Clin Exp Rheumatol 2008; 26: 632-637.
- Malemud CJ. Growth hormone, VEGF and FGF: involvement in rheumatoid arthritis. Clinica Chimica Acta Int J Clin Chem 2007; 375: 10-19.
- Saber T, Veale DJ, Balogh E, McCormick J, NicAnUltaigh S. Toll-like receptor 2 induced angiogenesis and invasion is mediated through the Tie2 signalling pathway in rheumatoid arthritis. PLoS One 2011; 6: e23540.
- Strunk J, Rumbaur C, Albrecht K, Neumann E, Muller-Ladner U. Linking systemic angiogenic factors (VEGF, angiogenin, TIMP-2) and Doppler ultrasound to anti-inflammatory treatment in rheumatoid arthritis. Joint Bone Spine Revue Du Rhumatisme 2013; 80: 270-273.
- Marotte H, Ahmed S, Ruth JH, Koch AE. Blocking ERK-1/2 reduces tumor necrosis factor alpha-induced interleukin-18 bioactivity in rheumatoid arthritis synovial fibroblasts by induction of interleukin-18 binding protein A. Arthritis Rheum 2010; 62: 722-731.
- Mainz ER, Serafin DS, Nguyen TT, Tarrant TK, Sims CE, Allbritton NL. Single cell chemical cytometry of akt activity in rheumatoid arthritis and normal fibroblast-like synoviocytes in response to tumor necrosis factor alpha. Analyt Chem 2016; 88: 7786-7792.
- Zhang W, Du Z, Zhu J, Yu J, Xu Y. Sprouty2 suppresses the inflammatory responses in rheumatoid arthritis fibroblast-like synoviocytes through regulating the Raf/ERK and PTEN/AKT signals. Mol Immunol 2015; 67: 532-539.
- Hensvold AH, Joshua V, Li W. Serum RANKL levels associate with anti- citrullinated protein antibodies in early untreated rheumatoid arthritis and are modulated following methotrexate. Arthritis Res Ther 2015; 17: 239.
- Omata Y, Tanaka S. RANKL/RANK signalLing in rheumatoid arthritis. Clin Calcium 2011; 21: 1175-1180.