Research Article - Biomedical Research (2017) Volume 28, Issue 6
Trimethoxy stilbene regulates the nasopharyngeal carcinoma cell apoptosis pathway and enhances sensitization to cisplatin-based chemotherapy
Hua Shao1#, Ruinian Zheng2# and Yueming Ding1*1Department of Otolaryngology, the First Affiliated Hospital of Dali University, Dali, Yunnan, China
2Department of Oncology, Dongguan People’s Hospital, Dongguan, Guangdong, China
#These authors contributed equally to this paper
- *Corresponding Author:
- Yueming Ding
Department of Otolaryngology
The First Affiliated Hospital of Dali University, China
Accepted date: November 2, 2016
Abstract
Objective: Nasopharyngeal carcinoma is a common malignant tumor. This study aims to explore the inhibitory effect and mechanism of trimethoxy stilbene on human nasopharyngeal carcinoma cells.
Methods: Human nasopharyngeal carcinoma cells, CNE1 and 5-8F, in logarithmic phase were collected and inoculated in a 96 pore cell culture plate. Cells were treated with 100 μl of culture media containing 2, 5, 10, or 20 mM of trimethoxy stilbene. MTT was added to each pore after 72 h of continuous cell culture. The absorbance value of each pore was detected at 490 nm by a micro plate reader. CNE1 and 5-8 F cells in logarithmic phase were inoculated in 6 pore cell culture plates. Cells were treated with 100 μl culture media containing 2, 5, 10, or 20 mM of trimethoxy stilbene. Cells cultured for two weeks or 24 h were stained with crystal violet. Photographic images were taken by a digital camera and recorded. Cell cloning was quantitatively analysed with quantity one (Bio-Rad). Annexin V-FITC and PT solution were added to the cell cultures. The mixture was then incubated under ambient temperature for 15 min.
Results: Cisplatin IC50 values of CNE1 and 5-8F cells processed with 20 μmol/L trimethoxy stilbene were evidently lower than that of the control group processed with 0 μmol/L trimethoxy stilbene. In addition, treatment with combined cisplatin and trimethoxy stilbene induced higher rates of apoptosis in CNE1 and 5-8F cells compared with only trimethoxy stilbene or cisplatin. The proportion of cells in G0/G1 phase increased as drug concentration increased. A concentration-effect relationship was observed, indicating that trimethoxy stilbene induced G2/M phase arrest in drug-resistant nasopharyngeal carcinoma cells.
Conclusion: Trimethoxy stilbene significantly inhibited the in-vitro proliferation capacity and clone formation ability of nasopharyngeal carcinoma cells. This inhibitory effect might be realized by inducing nasopharyngeal carcinoma cells to enter G2/M arrest.
Keywords
Trimethoxy stilbene, Nasopharyngeal carcinoma, Proliferation, Clone formation, Cell apoptosis.
Introduction
Nasopharyngeal carcinoma, a common malignant tumor, has shown become even more prevalent in recent years. At present, the main therapeutic method of nasopharyngeal carcinoma is radiotherapy. However, this treatment method has a high tumor recurrence rate, resulting in poor prognosis among patients [1].
In 1940, Resveratrol (Res) was obtained for the first time from Veratrum grandiflorum roots. The chemical name of resveratrol is trimethoxy stilbene. Research indicates that trimethoxy stilbene can regulate blood lipid metabolism, inhibit blood platelet aggregation, and provide protection from angiocarpy. It also has anti-inflammatory and anti-tumor effects. Several clinical tests had shown that trimethoxy stilbene inhibits malignant tumors [2]. In-vitro cell experiments had studied the influence of trimethoxy stilbene on proliferation, clone formation, and apoptosis of nasopharyngeal carcinoma cells, as well as further explored the specific mechanism underlying its inhibitory effects [3].
Materials and Methods
Experimental materials
Cell lines: Human nasopharyngeal carcinoma cell lines CNE1 and 5-8F were purchased from American Type Culture Collection.
Main experimental instruments: CO2 cell incubator (US Forma Scientific Corporation), clean bench top (Suzhou Cleaning Equipment Company), ultra-pure water purifier (Beijing Liuyi Instrument Plant), inverted microscope, and -80°C ultra-low temperature freezer (Japan SANYO Company).
Main reagent and materials: RPMI-1640 culture medium (US Gibco Corporation), DMEM culture medium (US Gibco Corporation), foetal bovine serum (Hangzhou Sijiqing Bioengineering Co., Ltd), trypsin, EDTA (US Gibco Corporation), trimethoxy stilbene (US Sigma-Aldrich Corporation), and MTT (US Sigma-Aldrich Corporation).
Experimental methods
Culturing cisplatin-resistant CNE1/DDP and 5-8F/DDP cell lines: Cisplatin-resistant CNE1/DDP and 5-8F/DDP cell lines were provided by West China Hospital, Sichuan University. Cells underwent 48 h of 10 μmol/L cisplatin processing every other 8 weeks to sustain drug resistance. Cells in logarithmic phase were collected for the experiment.
Detecting the influence of trimethoxy stilbene on the invitro proliferation of nasopharyngeal carcinoma cells by MTT method: CNE1/DDP and 5-8F/DDP cells in logarithmic phase were collected, digested, centrifuged, and re-suspended as a single-cell suspension. The cells were inoculated into 96 pore cell culture plates at a density of 5 × 103 cells/pore and incubated overnight in a cell incubator at 37°C and 5% CO2. By the following day, culture media in the pores had been absorbed. Then, 100 μl culture medium containing 2, 5, 10, or 20 mM trimethoxy stilbene was added to cells. The cells were continuously cultured for 72 h. Then, 20 μl MTT (5 mg/ml) was added to each pore. The supernatant was absorbed during 4 h of conventional culturing. The culture was oscillated for 10 minutes in the dark after the addition of 150 μl DMSO solution. The absorbance value of each pore was detected by a micro plate reader at 490 nm. Cell viability was calculated as (cell viability (%)=OD value in drug group/OD value in control group × 100%).
Detection of cell apoptosis with flow cytometry: CNE1/DDP and 5-8F/DDP cells in logarithmic phase were collected, conventionally digested, centrifuged, and re-suspended as single-cell suspensions. Cells were inoculated into a 6 pore cell culture plate at a density of 2 × 105 cells/pore and incubated overnight in a cell incubator at 37°C and 5% CO2. Culture media containing 2, 5, 10, or 20 mM of trimethoxy stilbene were replaced the following day. After 24 h of conventional culture, cells were digested with trypsin, centrifuged, and collected. Cells were washed twice with PBS and resuspended in 250 μl of buffer solution. Cell concentration was adjusted to approximately l × 106 cells/ml. A total of 100 μl cell suspension was collected and 5 μl Annexin V-FITC and 10 μl PI solution were added sequentially to the suspension. The suspension was incubated in the dark under ambient temperature for 15 minutes. Finally, 400 μl PBS of was added to the suspension. Cell apoptosis was detected and analysed by a flow cytometer.
Statistical analysis
Statistical analysis was conducted by IBM SPSS 17.0 software. All measurement data were expressed as x ± s. χ2 test was selected after comparing enumeration data. Group means were compared by t-test, one-way analysis of variance, SNK-q test, Spearman rank sum correlation analysis, multi-factor analysis, and dichotomy logistic regression analysis. P<0.05 indicated that the difference was statistically significant.
Results
Determining the drug resistance of cell lines
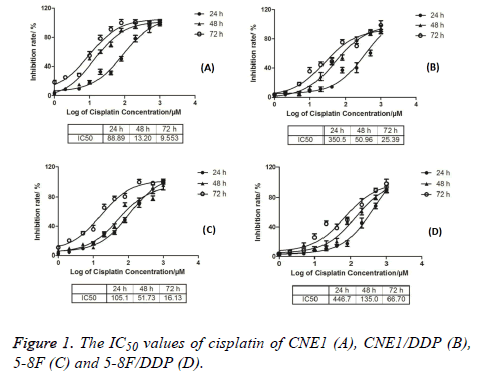
The IC50 values of CNE1, CNE1/DDP, 5-8F, and 5-8F/DDP treated with different cisplatin concentrations were detected by MTT method. The results are shown in Figure 1. The IC50 values of CNE1 cells at 24, 48, and 72 h were 88.89, 13.20, and 9.553 respectively. The IC50 values of CNE1/DDP cells at 24, 48, and 72 h were 350.5, 50.96, and 25.39 respectively. The IC50 values of 5-8F cells at 24, 48, and 72 h were 105.1, 51.37, and 16.13 respectively. The IC50 values of 5-8F/DDP cells at 24, 48, and 72 h were 446.7, 135.0, and 66.7, respectively, as shown in Figure 1.
Growth inhibition effect on CNE1/DDP cell line
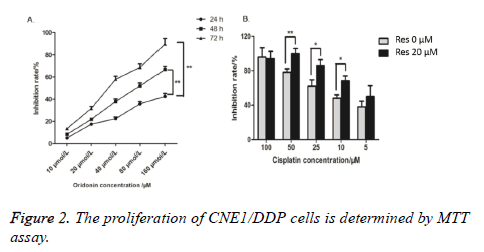
MTT method detected the inhibition ratios of CNE1/DDP cells processed with 10, 20, 40, 80 or 160 μmol/L trimethoxy stilbene for 24, 48, and 72 h. Figure 2A shows that the inhibition ratios of the CNE1/DDP cell line increased as trimethoxy stilbene concentration increased. Figure 2A also shows that under constant trimethoxy stilbene concentration, the inhibition ratio of CNE1/DDP increased as the acting time increased. Thus, a dosage-time relationship between trimethoxy stilbene concentration and inhibition ratio was observed. We also adopted MMT method to detect the inhibition ratios of the CNE1/DDP cell line processed with different cisplatin concentrations. Figure 2B shows that the inhibition ratio of the CNE1/DDP cell line processed with 20 μmol/L trimethoxy stilbene was evidently higher than that of the control group treated with 0 μmol/L trimethoxy stilbene. This result indicates that trimethoxy stilbene still had an inhibiting effect on drug-resistant nasopharyngeal carcinoma cell line.
Trimethoxy stilbene reduced the cisplatin IC50 values of CNE1/DDP and CNE1/DDP cell lines
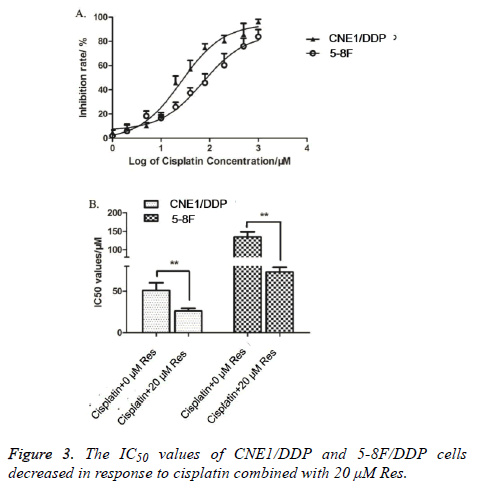
The inhibition ratios of CNE1/DDP and 5-8F/DDP cell lines treated with different trimethoxy stilbene concentrations were detected by MTT method. Figure 3A shows that the inhibition ratios of CNE1/DDP and 5-8F/DDP cell lines increased as trimethoxy stilbene concentration increased. Hence, a dosedependent relationship was observed between inhibition ratios and trimethoxy stilbene concentrations. We also detected the cisplatin IC50 values of the CNE1/DDP and 5-8F/DDP cell lines processed with 0 and 20 μmol/L of trimethoxy stilbene. Figure 3B shows that the cisplatin IC50 values of the CNE1/DDP and 5-8F/DDP cell lines treated with 20 μmol/L trimethoxy stilbene processing were obviously lower than those of the control group processed with 0 μmol/L trimethoxy stilbene. This result indicates that trimethoxy stilbene reduced the cisplatin IC50 values of CNE1/DDP and 5-8F/DDP cell lines.
Trimethoxy stilbene induced effect apoptosis of CNE1/DDP cells
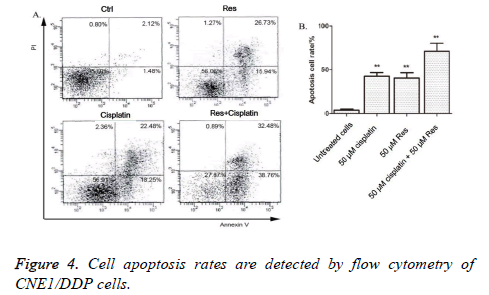
The cell apoptosis rates of CNE1/DDP cells processed with 50 μmol/L trimethoxy stilbene for 48 h were detected by flow cytometry. Figure 4 shows that that the combination of cisplatin and trimethoxy stilbene induced higher rates of apoptosis in CNE1/DDP cells, compared with independent trimethoxy stilbene or cisplatin treatment.
Discussion
Nasopharyngeal carcinoma, a malignant tumor with the highest morbidity and mortality rates in the world, is becoming more prevalent in various countries. Treatment methods for nasopharyngeal carcinoma include radiotherapy, chemotherapy, and immunotherapy, whereby chemotherapy plays an irreplaceable role. Although the comprehensive treatment of nasopharyngeal carcinoma has achieved enormous progress in recent years, nasopharyngeal carcinoma recurs in approximately 1/3rd of patients who received an initial treatment. One significant reason for recurrence is the drug resistance of tumor cells. Combined chemotherapy can exert cytotoxic effects to the greatest extent, but the occurrence of Multidrug Resistance (MDR) results in failure [4].
Heavy-metal drugs play an irreplaceable role in the chemotherapy of nasopharyngeal carcinoma. At present, heavy-metal drugs are used to extensively treat locally advanced and recurrent tumors, as well as metastatic nasopharyngeal carcinomas. Heavy-metal drugs improve therapeutic effects in nasopharyngeal carcinoma patients. Cisplatin is a representative of heavy-metal anti-tumor drugs. Cisplatin exerts anti-tumor effects through two main mechanisms: 1) intercalation between NDA bases to block the synthesis and transcription of DNA, and 2) inhibition of enzymatic activity, such as topoisomerase type II activity, to disintegrate genomic DNA [5]. Cisplatin is a periodic and nonspecific drug with killing effect on various tumor cells in logarithmic phase. However, cisplatin easily induces MDR of tumor cells in the clinical treatment of nasopharyngeal carcinoma. MDR is defined as the cross-drug resistance of tumor cells against different chemotherapeutics of other structures and functions generated by drug resistance against one kind of chemotherapeutics; therefore, MDR is a unique broad-spectrum drug resistance phenomenon [6]. Chemotherapy resistance is a major obstacle in the clinical treatment of idiopathic or acquired nasopharyngeal carcinoma. Treatment failure, recurrence, and metastasis of anaphase tumors result from MDR, seriously threatening the patient’s survival. Hence, there is an urgent need to discover effective drugs against MDR. Therefore, studying natural products with low toxicity and ability to reverse drug resistance has attracted increasing attention in recent years [7].
In the present study, we first detected the drug-resistant characteristics of the established human nasopharyngeal carcinoma cell lines, CNE1 and 5-8F, against cisplatin. Results showed that the CNE1/DDP and 5-8F/DDP cell lines were resistant to cisplatin compared with the CNE1 and 5-8F cell lines. To understand the effect of trimethoxy stilbene on drugresistant cells, the viability of CNE1/DDP cell lines processed with different concentrations of trimethoxy stilbene was detected by MTT method. Cell viability was compared with that of CNE1. The results indicate that the lethal effect of trimethoxy stilbene on CNE1/DDP cells was similar to that on CNE1. This result provides the initial foundation for the application of trimethoxy stilbene in late-stage refractory nasopharyngeal carcinoma.
At present, the mechanism of MDR is still unclear. Existing studies indicate that cells generate drug resistance by pumping out the drug before it exerts its cytotoxic effect, and by increasing drug metabolic enzymes, such as glutathione S transferase, to accelerate drug metabolism and form lowactivity metabolites [8], thus lowering the concentration and activity of topoisomerase type II in tumor cells. In addition, tumor cell resistance against apoptosis results in drug resistance. This study observed apoptosis in CNE1/DDP cells after 48 h of treatment with different trimethoxy stilbene concentrations. The results indicate that trimethoxy stilbene induced apoptosis in cisplatin-resistant cell lines of nasopharyngeal carcinoma and that apoptosis rate increased as the processing concentration of trimethoxy stilbene increased. The dosage-effect relationship indicates that trimethoxy stilbene terminated drug-resistant nasopharyngeal carcinoma cells by inducing cell apoptosis [9].
Apoptosis is the programmed death of multi-cellular organisms. Morphological changes associated with apoptosis include cytomembrane aleolation, cell shrinkage, chromatin concentration, nucleolus fragmentation, and apoptosis body formation [10]. Apoptosis maintains the steady state of organisms, and unbalanced apoptosis regulation causes various diseases, such as AIDS, neurodegenerative diseases, and malignant tumors. In recent years, inducing apoptosis has become the main mechanism of anti-tumor apoptosis for eliminating pre-cancer and cancer cells. A significant number of chemotherapeutic and chemoprophylaxis drugs exert their effects by inducing cell apoptosis. Therefore, apoptosis induction can be an index of screening new anti-tumor drugs and a method of evaluating the clinical efficacy of anti-tumor drugs. This study utilized flow cytometry to determine the cell apoptosis rates of nasopharyngeal cells processed with trimethoxy stilbene. The results showed that trimethoxy stilbene induced apoptosis and that apoptosis rates increased as the processing concentration of trimethoxy stilbene increased, which is the dosage-effect relationship. This result indicates that trimethoxy stilbene killed malignant tumor cells by inducing tumor cell apoptosis, and that apoptosis induction is an anti-tumor mechanism of trimethoxy stilbene.
Conclusion
Trimethoxy stilbene can inhibit the growth of MDR tumor cells aside from inhibiting the growth and metastasis of non-drugresistant tumors. Trimethoxy stilbene arrests cells in the G2/M phase of cell cycle, thus inhibiting the growth of drug-resistant nasopharyngeal carcinoma cells and inducing apoptosis. This study provides an idea on the application of trimethoxy stilbene, as well as the initial theoretical foundation for endstage refractory malignant tumor patients with MDR.
Acknowledgments
This work was supported by grants from the Social Development Science and Technology Plan of Yunnan Province (2010CD124).
References
- Lee N, Xia P, Quivey JM, Sultanem K, Poon I. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys 2002; 53: 12-22.
- Bai X, Guan B, Zhu Q. Antitumor and apoptosis induction effects of hederagenin on hepatocarcinoma (H22) tumor-bearing mice. Lat Am J Pharm 2016; 35: 1509-1518.
- Sultanem K, Shu HK, Xia P. Three-dimensional intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: the University of California-San Francisco experience. Int J Radiat Oncol 2000; 48: 711-722.
- Zhang L. The dietary flavonoid apigenin induces apoptosis and cell cycle arrest in nasopharyngeal carcinoma cells by targeting Akt-GSK-3ß-Cyclin D1 pathway. Lat Am J Pharm 2016; 35: 1192-1198.
- Zur Hausen H, Schulte-Holthausen H, Klein G, Henle W, Henle G. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature 1970; 228: 1056-1058.
- Lo YM, Chan LY, Lo KW, Leung SF, Zhang J. Quantitative analysis of cell-free Epstein-Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res 1999; 59: 1188-1191.
- Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 2006; 15: 1765-1777.
- Yu MC, Mo CC, Chong WX, Yeh FS, Henderson BE. Preserved foods and nasopharyngeal carcinoma: a case-control study in Guangxi, China. Cancer Res 1988; 48: 1954-1959.
- Kam MK, Teo PM, Chau RM, Cheung KY, Choi PH. Treatment of nasopharyngeal carcinoma with intensity-modulated radiotherapy: the Hong Kong experience. Int J Radiat Oncol Biol Phys 2004; 60: 1440-1450.
- Luo H, Cao R, Zhu L. Ampelopsin sodium induce apoptosis in human lung adenocarcinoma cell line SPC-A-1 in vitro. Lat Am J Pharm 2016; 35: 1594-1600.