Research Article - Journal of Clinical Pathology and Laboratory Medicine (2017) Journal of Clinical Pathology and Laboratory Medicine (Special Issue 2-2017)
Treatment of fetal growth restriction
- *Corresponding Author:
- Kazuo Maeda
Honorary Professor Department of Obstetrics and Gynecology Tottori University Medical School Yonago Japan
Tel: +81 857-31-5007
Fax: 81-859-22-6856
E-mail: maedak@mocha.ocn.ne.jp
Accepted date: December 19, 2016
Citation: Maeda K, Utsu M. Treatment of fetal growth restriction. J Clin Path Lab Med. 2017;1(2):9-11.
Abstract
Aim: Intrauteine treatment of fetal growth restriction (FGR).
Method: The mechanism to decrease villous transfer of nutrition followed by fetal asphyxia. Fetal was studied, and detected fibrin deposit in the placenta by the high gray level histogram width (GLHW) tissue characterization higher than normal placenta, then the fibrin deposit was soluted by maternal heparin injection in 17-31 gestational weeks.
Results: High GLHW reduced and estimated fetal weight increased to normal levels after heparin treatment. Normal neonate without asphyxia was born by caesarean delivery at 38 weeks.
Discussion: Also a fetus died after placental infarction caused by preeclampsia, which will be treated by the perioheral arterial anti-sympathicotonic therapy. Genetic FGR may receive genetic therapy in the future. Conclusion: Heparin treatment was effective to treat FGR caused by placental fibrin deposit. Other FGRs may be treated by their causative therapy
Keywords
Fetus; Growth restriction; Placenta; Fibrin deposit, Preeclampsia; Sympathicotony; Genetic disease.
Introduction
Retarded fetal growth should be treated in the uterus. Placental intervillous space fibrin deposit was a cause of FGR, showing estimated fetal weight decrease, and fetal asphyxia, which was the loss of variability and FHR decelerations within some weeks after the loss of FHR acceleration, resulting infantal demise [1]. A case of placental fibrin deposit was diagnosed by the high gray level histogram width (GLHW) value in the second trimester of pregnancy, then the mother was treated with heparin to solve fibrin deposit [2].
Research Methodology
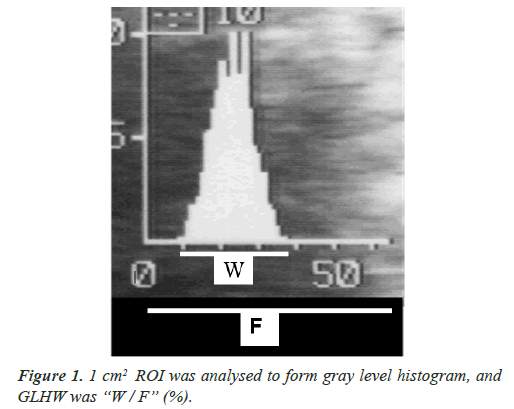
(GLHW) as clinical ultrasonic tissue characterization
As past ultrasonic tissue characterizations used particular computer and software, thus, its clinical application was difficult. Maeda [2] analyzed common gray level histogram of ultrasonic B-mode devices in 1 cm2 region of interest (ROI), where the histogram base width was divided by full gray scale of the histogram achieving GLHW value (%) (Figure 1). The GLHW of RMI 412 ultrasound phantom (Radiation Measurement Inc. Middleton, WI, USA) was studied, where the GLHW did not change when B-mode gain was controlled, while it was enlarged when the image contrast was high, thus, the GLHW was determined at the lowest image contrast. The phantom GLHW value was constant in various B-mode devices including a 3D machine. Clinical GLHW value was calculated manually, while it was automated by “%W” index in Aloka B-mode histogram, of which value was the same as manual determination [2].
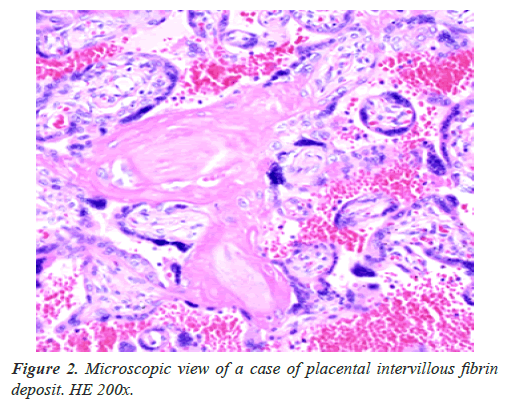
Fibrin deposit in placental intervillous space
Placental intervillous spatial blood flow was decreased by the deposited fibrin (Figure 2), then active transfer of villous function was restricted reducing nutritive materials, e,g. glucose and amino-acid to the fetus, reducing fetal weight, and restricting fetal growth, then plain villi transfer function of oxygen decreased developing fetal asphyxia after FGR, which lossed FHR acceleration against fetal movement forming nonreactive FHR in the actocardiogram (ACG) record, then severe fetal asphyxia and neonatal death appeared within some weeks after the loss of acceleration [1].
Fetal and neonatal outcome was ominous in FGR due to fetal asphyxia, while the FGR cases of normal FHR acceleration developed neither asphyxia nor fetal/neonatal damage [1]. Therefore, the reduction of placental fibrin deposit was intending normal fetal growth and preventing fetal/neonatal asphyxia with the reduction of placental fibrin deposit by maternal administration of heparin [2].
Diagnosis and treatment of placental fibrin deposit
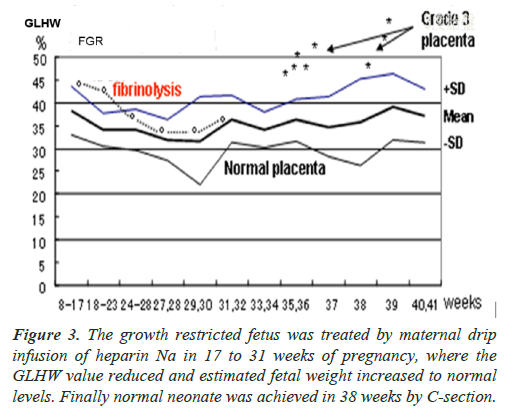
Placental B-mode image was white and the GLHW was as high as 43% in the case of second trimester FGR in 17 weeks of pregnancy, which was higher than normal placental GLHW (Figure 3) [2]. As cardiolipine antigen of the pregnant woman was positive, the fibrin deposit was confirmed in the intervillous space, and the fibrin was solved by the drip infusion of 5000 U heparin every day in 17-31 weeks of pregnancy.
Results
The GLHW decreased to 32% in 22 weeks of pregnancy, which was normal placental GLHW in 30 weeks (Figure 3), increasing estimated fetal weight to normal level, and a normal newborn without asphyxia was achieved in 38 weeks of pregnancy by C-delivery, because the fetus was very important baby.
FGR in pre-eclampsia
The author experienced a case of FGR followed by fetal death in a case of preeclampsia with placental infarction in 1960s, where the preeclampsia would be caused by the excitation of sympathetic nerve by the enlarging pregnant uterus [3-7]. Hypertension, proteinuria, arterial constriction, damages of various organs and fetus might be caused by the preeclampsia, i.e. placental maternal blood supply would be restricted by the uterine arterial constriction [9] due to the sympathicotony, inducing placental degeneration and infarction, where oxygen supply reduced to the fetus, resulting FGR, fetal asphyxia and death. Such high-risk fetus is cured by caesarean delivery from fetal asphyxia detected by FHR monitoring at present.
High uterine arterial resistance is detected by the loss of enddiastolic flow and reverse flow in pulsed Doppler uterine arterial flow resulting high resistance index, preceeding fetal asphyxia and postnatal neurological sequels at present [7].
Treatment of pre-eclampsia
Therefore, sympathetic excitation should be reduced by improve both maternal and fetal outcomes in preeclampsia 1) Stimulation of parasympathetic center, however: the method is unknown. 2) Use of parasympathetic drug, while the effect is unknown. 3) Uterus-brain nerve anesthesia by vaginal submucosal injection of anesthetics, while the effect is transient. 4) Surgically cut the uterus-brain nerve: it is contraindicated, because the nerve is physiologically important, e.g. regular labor contraction is formed by the positive feed-back loop including the nerve [8]. Therefore, medical treatment with antisympathetic agent should be invented in the future [9].
Discussion
There will be various causes of FGR, including hyponutrition of pregnant women. Maternal diseases also caused FGR. Fetal anomaly and diseases developed FGR; fetal congenital heart disease caused FGR due to deficient umbilical cord circulation. FGR was common in trisomy fetus. Thus, there may be some strategies to increase fetal growth. As the FGR fetus died in the previous pregnancy of the same mother without heparin therapy, an FGR suspectedly caused by placental fibrin deposit by ultrasound image and high GLHW [2], intrauterine treatment would be tried by fibrin solving heparin therapy, monitoring GLHW, estimated fetal weight and FHR.
Genetic fetal diseases usually develop symmetric FGR. Anomalous fetuses, e.g. 13, 18 and 21 trisomies are usually received symptomatic but not causative treatment; therefore, chromosomal engineering [10] and gene editing [11] will be studied for the treatment of genetic diseases in the future.
Conclusion
The FGR was treated solving fibrin deposit in placental intervillous space, which was detected by GLHW tissue characterization; Sympathetic nerve sedation will be the treatment of FGR in preeclampsia, and genetic disorders in the future.
References
- Teshima N. Non-reactive pattern diagnosed by ultrasonic Doppler fetal actocardiogram and outcome of the fetuses with non-reactive pattern. Acta Obset Gynecol. JPN 1993; 45: 423-430.
- Maeda K, Utsu M, Kihaile PE. Quanification of sonographic echogenicity with grey-level histogram width: a clinical tissue characterization. Ultrasound Med Biol. 1998; 24: 225-234.
- Maeda K. Preeclampsia is caused by continuous sympathetic center excitation due to an enlarged pregnant uterus. J Perinat Med. 2014; 42: 233-237.
- Wiesel O, Toth IE, Boldokoi Z, et al. Comparison of transsynaptic viral labeling of central nervous system structures from the uterine horn in virgin, pregnant, and lactating rats. Microsc Res Tech. 2004; 63: 244-252.
- Yellon SM, Grushan LA, Rambau GM, et al. Pregnancy-related changes in connections from the cervix to forebraiKn and hypothalamus in mice. Reproduction. 2010; 140: 155-164.
- Gnanamanickam GJ, Liewellyn-Smith IJ. Innervation of the rat uterus at esterus: a study in full-thickness, immune-peroxidase-stained whole-mount preparations. J Comp Neurol. 2011; 519: 621-643.
- Poletini MO, McKee DT, Szawa RE, et al. (2012) Cervical stimulation activates A1 and locus coeruleus neurons that project to the paraventricular nucleus of hypothalamus. Brain Res Bull. 2012; 88: 566-573.
- Maeda K. Uterine contractions in normal labor developed by a positive feed-back and oscllation. Health and Medical Informatics 2013; 4:3.
- Willliams Obsetrics. Hypertensive disorders- Pathogenesis ? (24th ed) Vasopasm. Mc Graw Hill, New York : 2014; p. 734.
- Kazuki Y, Kobayashi K, Aueviriyavit S, et al. Trans-chromosomic mice containing a human CYP3A cluster for prediction of xenobiotic metabolism in humans. Hum Mol Genet. 2013; 22: 578-92.
- Junges R, Khan R, Tovpeko Y, et al. Markerless genome editing in component Streptococci. Methods Mol Biol. 2017; 1537: 233-247.