Research Article - Biomedical Research (2017) Volume 28, Issue 7
Transforming growth factor β1 (TGF-β1) regulates the expression of extracellular matrix genes in a concentration-dependent manner
Rongfeng Zhang1*#, Jiagang Xu2#, Xiangkun Wu3 and Zhentai Liu2
1Department of Orthopedics, the 89th Hospital of People's Liberation Army, Weifang, Shandong, PR China
2Department of Pharmacy, the 89th Hospital of People's Liberation Army, Weifang, Shandong, PR China
3Department of Laboratory, the 89th Hospital of People's Liberation Army, Weifang, Shandong, PR China
#These authors contributed equally to this work.
- *Corresponding Author:
- Rongfeng Zhang
Department of Orthopedics, The 89th Hospital of People's Liberation Army
Shandong, PR China
Accepted date: December 07, 2016
Abstract
Here, we investigated the effects of different concentrations of transforming growth factor β1 (TGF-β1) on the regulation of aggrecan (ACAN), collagen type II (COL2A1), and SRY-box 9 (Sox9) gene expression in human nucleus pulposus cells in vitro. Second-generation human nucleus pulposus cells were grown in culture media containing 4 different concentrations of TGF-β1 (0.1, 1.0, 10, and 100 μg/L). The control group was not treated with TGF-β1. Then, reverse transcription polymerase chain reaction was used to measure the gene expression of ACAN, COL2A1, and Sox9. There were no significant differences in expression between the control group and the group treated with 0.1 μg/L TGF-β1. However, significant increases in the expression of ACAN, COL2A1, and Sox9 were observed as the concentration of TGF-β1 increased, and expression was highest following treatment with 10 μg/L TGF-β1. Thus, TGF-β1 induced the expression of ACAN, COL2A1, and Sox9 in nucleus pulposus cells, suggesting that TGF-β1 may play an important role in the prevention and treatment of intervertebral disc degeneration.
Keywords
TGF-β1, Nucleus pulposus cells, Gene expression, Aggrecan, Collagen type II, SRY-box 9.
Introduction
Disc degeneration is a major cause of common conditions such as cervical spondylosis and low-back pain [1]. Current therapies, including conservative treatments and invasive procedures, can only relieve the pain of disc degeneration, but cannot restore disc structure and function [2,3]. Therefore, effective therapies for intervertebral-disc degeneration are urgently needed. The extracellular matrix of the nucleus pulposus is composed of COL2A1 and proteoglycans, which function to withstand shearing force and conductive pressure. ACAN, the most common proteoglycan, plays an important role in maintaining disc height and withstanding compression [4]. In the process of degeneration, activation of matrix metalloproteinase and aggrecanase lead to a rapid decrease in matrix-like collagen II and ACAN; this causes marked damage to and degeneration of intervertebral discs [5]. Therefore, regeneration of the nucleus pulposus is of great interest for designing new therapies to maintain the function of the intervertebral disc [1,6].
Nucleus pulposus cells are the major matrix-secreting cell type. However, only a few of these cells exist in normal nucleus pulposus tissues, and they are easily differentiated in vitro, causing them to lose their normal cellular phenotype and limiting their application in biological therapy [7]. Therefore, stimulation of human nucleus pulposus cells to synthesize extracellular matrix and maintain the normal cell phenotype through the application of growth factors or gene therapy as a means to reverse disc degeneration is emerging as an interesting therapeutic approach [8]. SOX9, COL2A1, and ACAN could be identified based on expression level despite of their resemble phenotype in nucleus pulposus cells and articular chondrocytes [9]. The expression of these chondrogenic proteins and transcription factors has been widely characterized as the nucleus pulposus phenotype [10]. Transforming growth factor β1 (TGF-β1) is involved in cell proliferation and extracellular matrix synthesis, and these functions have been confirmed in both cartilage and endothelial cells [11]. In this study, we demonstrated the effects of TGF-β1 on the expression of ACAN, COL2A1, and Sox-9 in normal human nucleus pulposus cells to explore effective methods to stop or reverse early stage disc degeneration.
Materials and Methods
Cell collection and culture
The study protocol was approved by the ethics committee of our hospital. After obtaining written informed consent, nucleus pulposus samples were obtained from 3 donors with scoliosis who underwent anterior correction surgery. The donors were 8-19 years old, with a mean age of 13.8 years. Nucleus pulposus tissues were sealed in a sterile container, transported to the laboratory within 20 minutes, and screened immediately on a super clean bench. Then, the fibrous ring and adjacent tissues were removed with surgical scissors. The core nucleus pulposus tissues were separated from the vertebral cartilage under a microscope. Remnant tissues were washed twice with phosphate-buffered saline (PBS; HyClone, Logan, UT, USA) to remove debris, cut into pieces (1-2 mm2) with a scalpel, and digested with 0.25% tryptase (HyClone) at 37°C for 30 min, with shaking every 5 min. Next, the tissues were centrifuged at 1,000 × g for 5 min and then digested with 0.2% collagenase type II (Gibco-Invitrogen, USA) at 37°C for 4 h after removing the supernatant. Samples were filtered through a nylon screen and centrifuged at 1,000 × g for 5 min. The above procedures were repeated 3 times. Finally, the human nucleus pulposus cells were diluted in 2 mL of F12-10% FBS (HyClone) and counted using counting plates (Corning, NY, USA). The cells were seeded into culture bottles (Corning) at a density of 1 × 104 cells/mL in F12-10% FBS and incubated at 37°C and 5% CO2. The medium was changed every 3 days. Cells were grown until 80% confluence and then digested with 0.25% tryptase and split into 5 culture bottles for subculturing (the first passage). After reaching 80% confluence, cells were cultured and passaged again by the same method. Average cell viability was 96%, as detected by trypan blue staining. According to the regulations governing the administration of medical institutions issued by the state council of the PRC, patients were informed of the study protocol and related risks before this study, and they signed consent forms.
Test groups
Human nucleus pulposus cells from the second passage, grown to 80% confluence, were centrifuged at 1,000 × g for 5 min. The supernatant was removed, and F12 medium containing 10% fetal bovine serum (FBS; HyClone) was added to obtain a cell density of 1 × 104 cells/mL. The cells were split into 5 culture bottles. After 24 h incubation, the medium was removed, and 10% FBS/F12 medium containing 0.1, 1.0, 10, or 100 μg/L of TGF-β1 (Peprotech, Rocky Hill, NJ, USA) was added (the treatment groups). Medium without added growth factor was used for the control group.
Morphological observation of human nucleus pulposus cells
Cells from each group were observed by inverted phase contrast microscopy every day during this study, and differences in morphology and growth status were compared among groups.
Cell survival rate determination
Cells in all groups were cultured for 72 h and then digested with 0.25% tryptase. Then, the cell survival rate was determined by cell counting using trypan blue exclusion.
Total RNA extraction and reverse transcription polymerase chain reaction (RT-PCR)
Cells were cultured for 72 h and total RNA was extracted using TRIzol one-step (Gibco, Grand Island, NY, USA), according to the manufacturer’s instructions. RNA purity and concentration were assessed using an ultraviolet spectrophotometer. Next, 2 μg of total RNA was reverse transcribed with AMV revertase (Takara, Shiga, Japan) according to the manufacturer’s instructions. The cDNA for ACAN, COL2A1, and SOX-9 was amplified by real-time PCR. Primers were synthesized by Beijing Aoke Bio-Tech Co., Ltd. (Beijing, China), as previously described [8]. Glyceraldehyde phosphate dehydrogenase (GAPDH) was used as an internal reference. Table 1 shows the sequences of the primers for ACAN, COL2A1, and Sox9 [12]. The reaction conditions were 30 cycles of 3-stage PCR (denaturation at 94°C for 30 s, annealing at 52°C for 45 s, and extension at 72°C for 45 s). The amplified cDNA products were analyzed by 1.5% agarose gel electrophoresis. Electrophoresis photographs were analyzed using the IBAS2.5 image processing system. The absorbance ratios of ACAN, COL2A1, and Sox9 to GAPDH were used to determine the relative expression levels.
| Gene | Primer type | Sequence |
|---|---|---|
| Collagens type II | Primer fw (5’-3’) | AAG AAA CAC ATC TGG TTT GGA GAA A |
| Primer rev (5’-3’) | TGG GAG CCA GGT TGT CAT C | |
| Aggrecan | Primer fw (5’-3’) | CCA ACG AAA CCT ATG ACG TGT ACT |
| Primer rev (5’-3’) | GCA CTC GTT GGC TGC CTC | |
| SOX-9 | Primer fw (5’-3’) | AAC GCC GAG CTC AGC AAG |
| Primer rev (5’-3’) | ACG AAC GGC CGC TTC TC |
Table 1: Oligonucleotide primers used for real-time PCR.
Statistical analysis
Each experiment was repeated 3 times. Statistical analysis was performed using SPSS15.0 software (SPSS, Inc., Chicago, IL, USA), and the results are presented as the mean ± SD. Oneway analysis of variance was used to compare the control and treatment groups. The t-test was used to compare 2 groups. A P value less than 0.05 was considered statistically significant.
Results
Morphological observations
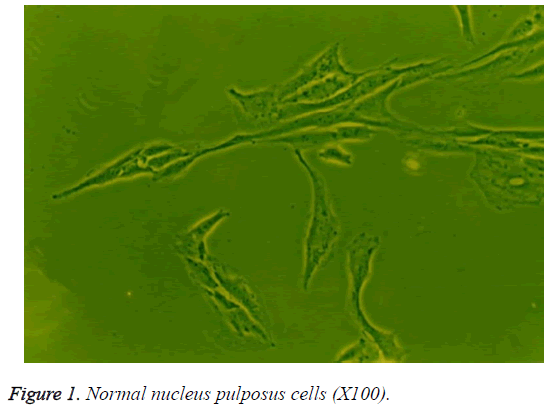
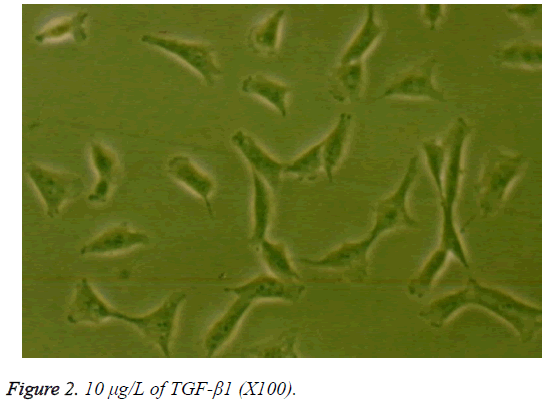
When grown in monolayer culture with medium containing 10% serum, the human nucleus pulposus cells in the control group were fusiform with 2 cytoplasmic processes (Figure 1). Compared to the control group, the cells in the treatment groups showed no significant morphological differences when cultured for less than 72 h. However, at 72 h, the cells in the groups treated with 10 or 100 μg/L TGF-β1 were short and spindle-shaped or polygon-shaped with short cytoplasmic processes, abundant cytoplasm, and powerful refractivity, compared to the those in the control group. The morphology of cells exposed to 10 μg/L TGF-β1 showed the greatest differences (Figure 2). There were no striking differences among the 0.1 μg/L TGF-β1, 1.0 μg/L TGF-β1, and control groups at 72 h.
Figure 1: HPLC chromatogram of the nine reference compounds in 50% aqueous methanol, measured at 370nm. Retention times for rutin, sutherlandin A, sutherlandin B, kaempferol-3-O-rutinoside, sutherlandin C, sutherlandin D, quercitrin, quercetin and kaempferol were 11.9, 12.7, 13.8, 15.3, 16.2, 17.0, 18.0, 26.2 and 28.1 minutes, respectively.
Figure 2: HPLC chromatogram of the nine reference compounds in 50% aqueous methanol, measured at 370nm. Retention times for rutin, sutherlandin A, sutherlandin B, kaempferol-3-O-rutinoside, sutherlandin C, sutherlandin D, quercitrin, quercetin and kaempferol were 11.9, 12.7, 13.8, 15.3, 16.2, 17.0, 18.0, 26.2 and 28.1 minutes, respectively.
Cell survival
In the presence of 10% FBS, the survival rate of cells in the control group was 89%. Exposure to TGF-β1 increased the cell survival rate, and the cell survival rates of the 0.1 μg/L, 1.0 μg/L TGF-β1, and 10 μg/L TGF-β1 groups were 91%, 94%, and 96%, respectively. However, the cell survival rate decreased to 93% at the highest concentration of TGF-β1 (100 μg/L).
Effects of TGF-β1 on the gene expression of ACAN, COL2A1, and SOX-9
The absorbance ratios of ACAN to GAPDH in the control and 0.1 μg/L TGF-β1 groups were similar at 0.775 ± 0.024 and 0.828 ± 0.008, respectively. The absorbance ratios of ACAN to GAPDH in the 1 μg/L, 10 μg/L, and 100 μg/L TGF-β1 groups, at 1.361 ± 0.016, 1.814 ± 0.029, and 1.663 ± 0.027, respectively, were significantly higher than that in the control group (P<0.05). Similarly, the absorbance ratio of COL2A1 to GAPDH was 1.312 ± 0.036 in the control group, which was not significantly different from that of the 0.1 μg/L TGF-β1 group. However, compared to the control group, the COL2A1 to GAPDH ratios of the other TGF-β1 treatment groups were obviously increased. The most striking effect of TGF-β1 treatment was observed in the 10 μg/L TGF-β1 group, in which the COL2A1 to GAPDH ratio was 2.18 times higher than that of the control group (P<0.05). Additionally, there was no significant difference in Sox-9 gene expression between the control group and the 0.1 μg/L TGF-β1 group (P>0.05), whereas there was a significant difference between the control group and the 1 μg/L TGF-β1 group (P<0.05). Compared to the control group, Sox-9 gene expression levels in the 10 and 100 μg/L TGF-β1 groups were significantly higher (P<0.01). These data showed a marked elevation in Sox-9 gene expression following exposure to 10 μg/L TGF-β1 when compared to the expression level in the control group (P<0.01). However, the gene expression of ACAN, COL2A1, and SOX-9 showed a tendency to decrease in response to higher TGF-β1 concentrations (Table 2).
| TGF-β1 group | Aggrecan | Collagen type II | SOX-9 |
|---|---|---|---|
| Control group | 0.775±0.024 | 1.312±0.036 | 1.186±0.019 |
| 0.1μg/L group | 0.828±0.008 | 1.497±0.022 | 1.247±0.035 |
| 1.0μg/L group | 1.361±0.016* | 1.939±0.031* | 2.352±0.031* |
| 10μg/L group | 1.814±0.029** | 2.862±0.038** | 3.374±0.056** |
| 100μg/L group | 1.663±0.027** | 2.715±0.024** | 3.161±0.037** |
Table 2: The mRNA expressions of Aggrecan, collagen type II and SOX-9 (n=3, ?x ± s).
Discussion
Degenerative disc disease has high rates of incidence and disability and frequently causes neck, shoulder, low-back, and leg pain. Nucleus pulposus tissues play a primary role in this disease [13]. Normal nucleus pulposus tissue is tremellose composed of proteoglycan and COL2A1. Compared to cartilage tissue, there is more proteoglycan in nucleus pulposus tissues, and the ratio of proteoglycan to COL2A1 is 27:1 in the nucleus pulposus and 2:1 in cartilage [14]. Within the nucleus pulposus, proteoglycan is embedded in grids composed of COL2A1. ACAN, the main proteoglycan component, shows powerful adsorption ability, and the water content in normal pulposus tissues is as high as 80%, which imparts various characteristics, such as flexibility, viscous-elastic behavior, crushing resistance, and shock absorption [15]. Because there is no vascular tissue in the nucleus pulposus, nutrition is supplied through diffusion and dispersion from adjacent tissues [16], which makes the nucleus pulposus prone to degeneration when subjected to pressure for an extended time period. During disc degeneration, ACAN and COL2A1 levels decrease, while collagen type I (COL1A2) levels increase, which promotes gradual fibrosis and degeneration of the fibrous ring and cartilage endplate.
Current therapies for degenerative disc disease include conservative treatment and surgery. However, these methods only relieve the symptoms but do not eliminate the cause of disc degeneration, and the long-term effects are not ideal [17]. With the advent of tissue engineering and regenerative medicine, cell-based therapies for the repair of degenerative discs have gained attention [18,19]. Cytokines are used to improve the activity of nucleus pulposus cells and promote the synthesis of normal extracellular matrix, enabling the reversal of disc degeneration. TGF-β1 is a 2-chain polypeptide with a molecular weight of 25 kDa. It is a secreted protein that performs many cellular functions, including control of cell proliferation and cell differentiation, and promotion of extracellular matrix synthesis [20]. Vadala et al. [21] used a scaffold and directly incorporated TGF-β1 into a polymeric solution to culture bovine annulus fibrosus cells. After 3 weeks, glycosaminoglycan and total collagen levels were significantly higher in the treatment group than in the control group, indicating that TGF-β1 could be used to treat disc degeneration.
A previous study showed that TGF-β1 could promote cell proliferation in the presence of 10% serum, and there was a dose-effect relationship from 1 to 10 μg/L TGF-β1 [22]. Because nucleus pulposus cells lack specific cell markers, some studies evaluated ACAN, COL2A1, and Sox-9 expression as specific markers. Sox-9 promotes cartilage cell differentiation and maintains the cartilage cellular phenotype, which is closely related to disc degeneration. Sox-9 increases the synthesis of ACAN and COL2A1 in the disc matrix [23].
Our results showed that TGF-β1, at 1-100 μg/L, clearly promoted the expression of ACAN, COL2A1, and SOX-9. These results confirm that TGF-β1 can promote and maintain the expression of the nucleus pulposus cell phenotype in vitro, and 10 μg/L TGF-β1 showed the strongest effects.
The mechanisms of TGF-β1 activity are unclear; thus, studies examining the effect of TGF-β1 on the expression of genes in the extracellular matrix of the nucleus pulposus are needed. First, TGF-β1, which promotes the expression of proteoglycan and COL2A1, should be directly injected into degenerative nucleus pulposus tissues to improve the activity and proliferation of nucleus pulposus cells. However, some researchers have suggested that since growth factors have short half-lives and disc degeneration is a chronic process, it is difficult to obtain satisfactory effects through injection [24]. Interestingly, some studies have found that proteoglycan and COL2A1 levels in discs were increased when TGF-β1 was injected into a degenerative disc model (P<0.01) [25]. These results indicated that TGF-β1 promotes the synthesis of extracellular matrix, thus delaying disc degeneration. Additionally, fabrication of a tissue-engineered injectable scaffold to treat degenerative disc disease may be possible [26,27]. Nucleus pulposus cells and stem cells cultured in vitro were used as seed cells. Stem cells are common and show multi-directional differentiation; however, an effective method for differentiating stem cells into nucleus pulposus cells has not been established. Furthermore, the number of nucleus pulposus cells is limited, and they show low activity. The results of this study indicate that in nucleus pulposus cells cultured in vitro, TGF-β1 promotes the expression of the normal cell phenotype, making it possible to culture nucleus pulposus cells in vitro for use as seed cells in disc engineering.
Authors’ Contribution
Dr. Rongfeng Zhang performed the experiments and prepared the manuscript. Dr. Rongfeng Zhang and Dr. Jia-gang Xu took charge of the experimental design. Dr. Xiang-kun Wu and Zhen-tai Liu helped assess the experimental design and prepare the manuscript.
Acknowledgements
This study was supported by National Natural Science Foundation of China (30370389).
References
- Buchbinder R, Blyth FM, March LM, Brooks P, Woolf AD. Placing the global burden of low back pain in context. Best Pract Res Clin Rheumatol 2013; 27: 575-589.
- Arkesteijn IT, Smolders LA, Spillekom S, Riemers FM, Potier E, Meij BP, Ito K, Tryfonidou MA. Effect of coculturing canine notochordal, nucleus pulposus and mesenchymal stromal cells for intervertebral disc regeneration. Arthritis Res Ther 2015; 17: 60.
- Sun Z, Luo B, Liu ZH, Samartzis D, Liu Z, Gao B, Huang L, Luo ZJ. Adipose-Derived Stromal Cells Protect Intervertebral Disc Cells in Compression: Implications for Stem Cell Regenerative Disc Therapy. International J Biol Sci2015; 11: 133-143.
- Feng Y, Egan B, Wang J. Genetic Factors in Intervertebral Disc Degeneration. Genes Dis 2016; 3: 178-185.
- Hu X, Zhou Y, Zheng X, Tian N, Xu C, Wu W. Differentiation of menstrual blood–derived stem cells toward nucleus pulposus-like cells in a coculture system with nucleus pulposus cells. Spine 2014; 39: 754-760.
- He F, Pei M. Rejuvenation of nucleus pulposus cells using extracellular matrix deposited by synovium-derived stem cells. Spine 2012; 37: 459-469.
- Wu J, Wang D, Ruan D, He Q, Zhang Y, Wang C, Xin H, Xu C, Liu Y. Prolonged expansion of human nucleus pulposus cells expressing human telomerase reverse transcriptase mediated by lentiviral vector. J Orthop Res 2014; 32: 159-166.
- Sakai D, Nakai T, Mochida J, Alini M, Grad S. Differential phenotype of intervertebral disc cells: microarray and immunohistochemical analysis of canine nucleus pulposus and anulus fibrosus. Spine 2009; 34: 1448-1456.
- Guan X, Ma X, Zhang L, Feng H, Ma Z. Evaluation of CD24 as a marker to rapidly define the mesenchymal stem cell phenotype and its differentiation in human nucleus pulposus. Chin Med J (Engl) 2014; 127: 1474-1481.
- Sun Y, Lv M, Zhou L, Tam V, Lv F, Chan D. Enrichment of committed human nucleus pulposus cells expressing chondroitin sulfate proteoglycans under alginate encapsulation. Osteoarthritis and Cartilage 2015: 1194-1203.
- Tran CM, Smith HE, Symes A, Rittié L, Perbal B, Shapiro IM, Risbud MV. Transforming growth factor ß controls CCN3 expression in nucleus pulposus cells of the intervertebral disc. Arthritis Rheum 2011; 63: 3022-3031.
- Li Z, Kaplan KM, Wertzel A, Peroglio M, Amit B. Biomimetic fibrin-hyaluronan hydrogels for nucleus pulposus regeneration. Regen Med 2014; 9: 309-326.
- Siemionow K, An H, Masuda K, Andersson G, Cs-Szabo G. The effects of age, sex, ethnicity, and spinal level on the rate of intervertebral disc degeneration: a review of 1712 intervertebral discs. Spine2011; 36: 1333-1339.
- Ruan D, Zhang Y, Wang D, Zhang C, Wu J, Wang C, Shi Z, Xin H, Xu C, Li H, He Q. Differentiation of human Wharton’s jelly cells toward nucleus pulposus-like cells after coculture with nucleus pulposus cells in vitro. Tissue Eng 2012; 18: 167-175.
- Woods BI, Vo N, Sowa G, Kang JD. Gene therapy for intervertebral disk degeneration. Orthop Clin North Am 2011; 42: 563-574.
- Woods BI, Sowa G, Vo N, Kang JD. A Change in strategy: the use of regenerative medicine and tissue engineering to augment the course of intervertebral disc degeneration. Oper Tech Orthop 2010; 20: 144-153.
- Wan Y, Feng G, Shen FH, Laurencin CT, Li X. Biphasic scaffold for annulus fibrosus tissue regeneration. Biomaterials 2008; 29: 643-652.
- Chan LK, Leung VY, Tam V, Lu WW, Sze KY. Decellularized bovine intervertebral disc as a natural scaffold for xenogenic cell studies. Acta Biomater 2013; 9: 5262-5272.
- Xu B, Xu H, Wu Y, Li X, Zhang Y, Ma X, Yang Q. Intervertebral Disc Tissue Engineering with Natural Extracellular Matrix-Derived Biphasic Composite Scaffolds. PLoS One 2015; 10: e0124774.
- Hsieh HL, Wang HH, Wu WB, Chu PJ, Yang CM. Transforming growth factor-β1 induces matrix metalloproteinase-9 and cell migration in astrocytes: roles of ROS-dependent ERK- and JNK-NF-κB pathways. J Neuroinflammation 2010; 7: 88.
- Vadalà G, Mozetic P, Rainer A, Centola M, Loppini M. Bioactive electrospun scaffold for annulus fibrosus repair and regeneration. Eur Spine J 2012; 21 Suppl 1: S20-26.
- Zhang R, Ruan D, Zhang C. Effects of TGF-beta1 and IGF-1 on proliferation of human nucleus pulposus cells in medium with different serum concentrations. J Orthop Surg Res 2006; 1: 9.
- Gruber HE, Norton HJ, Ingram JA, Hanley EN Jr. The SOX-9 transcription factor in the human disc: decreased immunolocalization with age and disc degeneration. Spine (Phila Pa 1976) 2005; 30: 625-630.
- Okada M, Kim JH, Hutton WC, Yoon ST. Upregulation of intervertebral disc-cell matrix synthesis by pulsed electromagnetic field is mediated by bone morphogenetic proteins. J Spinal Disord Tech 2013; 26: 167-173.
- Wu B, Zhang H, Wang HB, Jia CL, Zhao YF. Effects of intervertebral injection of transforming growth factor beta 1 on proteoglycan expression in rabbit degenerated lumbar discs. J Clin Rehab Tissue Eng Res 2011; 15: 1961-1964.
- Foss BL, Maxwell TW, Deng Y. Chondroprotective supplementation promotes the mechanical properties of injectable scaffold for human nucleus pulposus tissue engineering. J Mech Behav Biomed Mater 2014; 29: 56-67.
- Wiltsey C, Kubinski P, Christiani T, Toomer K, Sheehan J, Branda A, Kadlowec J, Iftode C, Vernengo J. Characterization of injectable hydrogels based on poly(N-isopropylacrylamide)-g-chondroitin sulfate with adhesive properties for nucleus pulposus tissue engineering. J Mater Sci Mater Med 2013; 24: 837-847.