Review Article - Journal of Brain and Neurology (2017) Journal of Brain and Neurology (Special Issue 1-2017)
Transcranial Magnetic Stimulation (TMS) as a treatment tool in schizophrenia: A review.
- *Corresponding Author:
- Graziano B
Department of Psychaitry University of Pittsburgh Pittsburgh, USA
E-mail: grazianob@upmc.edu
Accepted date: November 14, 2017
Citation: Graziano B, Kaskie RE, Ferrarelli F. Transcranial Magnetic Stimulation (TMS) as a treatment tool in schizophrenia: A review. J Brain Neurol 2017;1(1):14-24.
Abstract
Schizophrenia is a chronic, debilitating psychiatric disorder that has an immense impact on the patients, their families, and the entire society. Schizophrenia is characterized by positive symptoms (e.g., hallucinations, delusions), negative symptoms (e.g., apathy, reduced emotional response), as well as a variety of cognitive dysfunctions. While positive symptoms are often successfully treated with antipsychotic medications, negative symptoms and cognitive impairments tend to be more treatment refractory. Moreover, the occurrence of persistent, systemic side effects in schizophrenia patients taking antipsychotic medications significantly affects their compliance. Thus, more targeted, symptom-specific treatment interventions are critically needed in schizophrenia. Transcranial Magnetic Stimulation (TMS) allows to directly and noninvasively reach the cortical surface. TMS can therefore target specific cortical areas, which are involved in some of the symptoms or cognitive dysfunctions commonly experienced by schizophrenia patients, while at the same time minimizing systemic side effects. Furthermore, it has been shown that repetitive stimulation (rTMS) delivered at low frequency (≤ 1 Hz) or high frequency (>1 Hz) can respectively increase or decrease neural excitability, including the brain regions involved in the pathophysiology of schizophrenia. Because of these unique features, over the past two decades there has been a burgeoning of studies investigating rTMS as a treatment tool in schizophrenia. In this review, we will systematically analyse these data, focusing on studies that examined the effects of rTMS on positive, negative and cognitive symptoms. Specifically, we will present main findings from these rTMS studies, highlight their strengths and pitfalls, and we will then discuss how these findings may contribute to develop more effective, TMS-based treatment interventions in schizophrenia.
Keywords
Schizophrenia, Psychiatric disorder, Cognitive dysfunction, Transcranial magnetic stimulation.
Introduction
Schizophrenia is a severe and chronic psychiatric disorder which affects 0.4%-1% of the population worldwide [1]. Schizophrenia has an important economic impact, both on individual patients and on the entire community, and is characterized by a multitude of symptoms that can be clinically distinguished in positive, negative and cognitive symptoms [2,3]. Since the serendipitous discovery of the first antipsychotic medication (chlorpromazine) in the 1950s, numerous other pharmacological compounds have been developed for schizophrenia [4]. Thanks to these medications, the management of symptomatology of schizophrenia and related disorders has achieved significant results, particularly in relation to treatment of positive symptoms, whereas they are far less effective for managing negative symptoms and cognitive dysfunctions [5-7]. Moreover, since antipsychotic medications do not have a specific mechanism of action, but operate more on a systemic level, a substantial number of patients report significant, persistent side effects [5]. Therefore, more specific and localized interventions have been investigated to ameliorate treatment interventions in schizophrenia.
Transcranial Magnetic Stimulation (TMS) is a neurophysiological technique that utilizes a magnetic field to influence membrane potentials of cortical neurons. This non-invasive procedure is capable of directly evoking cortical neural potentials, which can then be recorded on the scalp using high-density electroencephalography, a characteristic that makes TMS a unique instrument for investigating the neurophysiological abnormalities of schizophrenia and other psychiatric disorders [6]. Moreover, since TMS is capable of altering membrane neural potentials, TMS can be used to modulate cortical excitability and, as a result, affect neural networks that are impaired in psychiatric conditions [8].
Recently, many studies have investigated the use of TMS as a treatment tool, because of several distinctive features. First, it is not-invasive and well-tolerated; the patient is awake during the whole treatment and no anesthesia is required. Only mild side effects are usually reported, like headaches and scalp pain, whereas the induction of seizure is extremely rare, especially when following safety guidelines. Second, unlike pharmacological intervention, no systemic side effects are usually observed [9]. Third, TMS can directly stimulate discrete cortical regions, which allows targeting specific brain networks and symptoms. Lastly, repetitive TMS (rTMS) can cause alterations in cortical excitability beyond the stimulation period, thus suggesting a therapeutic potential for such intervention [10].
rTMS consists of trains of magnetic pulses, which are able to promote cortical functional reorganization. Intensity, frequency and total number of pulses are stimulation variables that influence direction and degree of cortical plasticity [11]. In particular, high frequency rTMS (>1 Hz) appears to increase cortical excitability, while low frequency stimulation (≤ 1 Hz) seems to reduce it [12]. Therefore, rTMS is supposed to induce durable potentiation or depression in neural synapses, which may lead to lasting effects in cortical function [11]. Even though the therapeutic mechanism of rTMS has still to be fully elucidated, one of the most studied process underlying rTMS efficacy is the increased dopamine release in subcortical and cortical areas of the brain after rTMS treatment, which has been found in both animal and human studies [13-15]. Finally, rTMS has been employed as a treatment tool for many psychiatric conditions, including depression, obsessive compulsive disorder and post-traumatic stress disorder [8,16].
In this article, we will review studies investigating the effectiveness of rTMS in treating all categories of schizophrenia symptoms. We will present the main findings from these studies, which employed different stimulation patterns and targeted various cortical regions. Finally, we will discuss the implications of these findings in the treatment of schizophrenia.
Methods
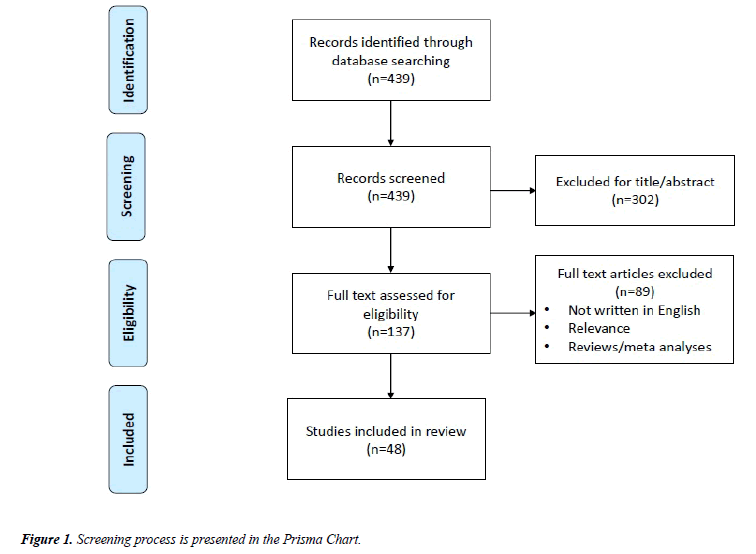
We used the search terms “(TMS OR Transcranial Magnetic Stimulation) AND (schizophreni*) AND (treatment OR therapy)” to conduct an online literature search using the MEDLINE database (1996–2017). Our screening process is presented in the Prisma Chart of Figure 1. First, articles were selected based on their title and abstract and we excluded articles whose primary focus was to evaluate neurophysiological alterations in schizophrenia as well as studies that were not related to schizophrenia, or that were not written in English. We also removed case studies and studies with small sample sizes (N<15), since we aimed to collect the findings of studies using moderate to large samples of patients. Moreover, since we organized the articles based on the treatment of specific group of symptoms (positive, negative and cognitive), we excluded those articles that did not specifically address the treatment of a group of symptoms or cognitive dysfunctions. Major findings from these selected studies, including stimulation parameters and targeted cortical area, are reported in Table 1. Furthermore, the efficacy of rTMS treatment was compared to sham or a different active stimulation protocol, and both primary and secondary outcomes were reported whenever available (see Table 1 for a summary of these findings).
| Symptoms | References | Sample | Stimulation | Active/Sham (A/S) | Additional Findings |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Auditory Verbal Hallucinations (AVH) | [21] | SZ/SAD=24 | 1 HzB; left TPC; 9 ss | HCS: ↓ A;=S | Significant decrease in hallucination frequency and attentional salience of hallucinations in the active group over time. |
| [33] | SZ/SAD=16 | 1 HzB; left TPC; 4 ss | PANSS-HS: ↓A; ↓ S | ||
| [34] | SZ=39 | 1 HzB; left/right TPC; 10 ss | AHRS:=A;=S | Left and right active rTMS resulted in better global CGI-I, PANSS-HS and frequency of AVH compared to the sham group. | |
| [22] | SZ=16 | 1 HzB; left TPC; 4 ss | SAH: ↓ A;=S (over time) | Over time and long term improvement in SAPS and SANS scores only in active rTMS group. | |
| [23] | SZ/SAD=51 | 1 HzB; left TPC; 9 ss | HCS: ↓↓ A; ↓ S | CGI was significantly improved in the active, compared with sham, rTMS group following treatment. | |
| [39] | SZ/SAD=33 | 1 HzB; left TPC; 10 ss | HCS: ↓A; ↓ S | ||
| [24] | SZ=24 | 1 HzB; left TPC; 5 ss | AHRS: ↓ A;=S | SAPS did not improve neither in active or in sham stimulation group. | |
| [25] | SZ=40 | 1 HzA; left TPC; 10 ss | AHRS: ↓ A;=S | ||
| [40] | SZ=18 | 1 HzA; left/right TC/COS; 1 ss each site | AHRS:=A;=S | Decrease of MADRS in active stimulation group. | |
| [35] | SZ=36 | 1 HzB; left/bilateral TPC; 6 ss | AHRS: ↓ A; ↓ S | Bilateral rTMS resulted in greatest self-reported improvement of AVH. Hallucination frequency was significantly reduced only in the left rTMS group. | |
| [36] | SZ=18 | 1 HzB; left TPJ; 6 ss | PANSS-HS:=A;=S | ||
| No significant difference between groups in PANSS-HS. | |||||
| [37] | PP=62 | 1 HzB; left TPC (AVH-RAR); 15 ss | AHRS: ↓ A; ↓ S | ||
| Significant reduction in BPRS scores in active group compared to control group. | |||||
| [38] | SZ=17 | 1 HzB; left TPC; 20 ss | AHRS:=A;=S | ||
| In reducing AVH-severity stimulation of TPCs had same effectiveness of control-site. | |||||
| [26] | SS=24 | 1 HzA; left/right TPC/COS; 1 ss each site | AHRS: ↓ A; ↓S | ||
| Significant blood flow reduction in primary auditory cortex, left Broca's area and cingulate gyrus only in active stimulation group. No significant difference between the two protocols. | |||||
| [20] | SZ/SAD=30 | 1 Hz/TBSB; Spt Area; 10 ss | AHRS: ↓ A;=S | ||
| Before TMS difference in left STG blood flow between responders and non-responders. Association with response to TMS. No difference in improvement or response between the two protocols. | |||||
| [41] | SZ/SAD=24 | 1 Hz/TBSA; Spt Area; 10 ss | AHRS: - | TBS had equal clinical effects compared to 1Hz TMS. | |
| Improved HCS in patients whose motor threshold was detected consistently. Improvement in CGI and AVH frequency. | |||||
| [42] | SZ/SAD=24 | 1 Hz/TBSA; Spt Area; 10 ss | AHRS/PANSS: - | ||
| No difference between left and bilateral stimulation. Reduction of AHRS over time on each group. | |||||
| [28] | SZ/SAD=83 | 1 HzB; W/right W; 15 ss | HCS: ↓A; ↓S | ||
| Significant reduction in AVH after week 1 of treatment in both protocols. No superiority of treatment type. | |||||
| [27] | SZ=47 | 1 HzB; left/bilateral TPJ; 6 ss | PANSS-HS:=A;=S | ||
| [43] | SZ=18 | 1 Hz/20 HzA; AVH-RAR; 8 ss | AHRS: ↓ | ||
| No difference between protocols, except for AVH loudness in priming group at follow up. | |||||
| [29] | SZ/SAD=22 | Bilat 1Hz TPC/20Hz TPC/20 Hz BrocaB ; 3-5 ss | AHRS/HCS: ↓ A; ↓ S | ||
| Temporal Scalp to Cortex Distance and temporal and primary hand motor cortex Grey Matter Density predicted treatment efficacy. Reduction of AVH was greater when the distance from cortical temporal region was shorter. | |||||
| [44] | SZ=40 | 1 HzC; left TPC; 10 ss | AHRS: ↓ | ||
| Reduction of PANSS positive and general subscales in all groups. | |||||
| [46] | SZ=15 | 20 HzA; left temporal lobe; 2 ss | AHRS: - | ||
| No significant difference between sham and active stimulation group in BPRS or CGI-S. | |||||
| [30] | PP=64 | TBSB; left TPC; 10 ss | AHRS: ↓ A; ↓ S | More improved patients with external AVH than patients with internal AVH. | |
| [31] | SZ=30 | 20 HzB; left TPJ; 2 ss | AHRS: ↓ A; ↓ S | ||
| [32] | SZ=27 | 1 HzB; LPA; 10 ss | SAPS: ↓ A; ↓ S | ||
| Negative symptoms (NS) | [58] | SZ=20 | 10 HzB; left DLPFC; 10 ss | PANSS-NS: ↓↓ A; ↓ S | |
| [56] | SZ/SAD=20 | a /3 Hz/20 HzB; bilat DLPFC; 10 ss | PANSS-NS: ↓↓ A (a); ↓ S | Individualized a - TMS was associated with a significant reduction of PANSS-NS relative to the other 3 conditions. | |
| [62] | SZ=16 | 20 HzB; left DLPFC; 10 ss | PANSS-NS: ↓ A; ↓ S | Association of Sham TMS with improvement over time on the positive and negative subscales of the PANSS and MADRS. | |
| [63] | SZ=17 | 10 HzB; left DLPFC; 10 ss | PANSS-NS:=A;=S | No significant change in negative symptoms (immediately or 2 weeks post TMS). | |
| [57] | SZ=22 | 10 HzB; left DLPFC; 15 ss | PANSS-NS: ↓↓ A; ↓ S | Significant decrease in SANS score in active stimulation group. | |
| [64] | SZ/SAD=15 | 10 HzB; bilateral PFC; 15 ss | SANS:=A;=S | No significant changes in total PANSS score or CDSS score were found. | |
| [55] | SZ=48 | 1 Hz/10 HzB; left DLPFC; 20 ss | SANS: ↓ A (10 Hz);=S | No significant change in the 1 Hz or placebo rTMS groups in SANS score. | |
| [59] | SZ/SAD=25 | 20 HzB; bilateral DLPFC, 20 ss | SANS:=A;=S | No improvement in CDSS after TMS. | |
| [49] | SZ=40 | 10 HzB; left DLPFC; 15 ss | SANS: ↓↓ A; ↓ S | ||
| [50] | SZ=93 | 10 Hz/20 Hz/TBSB; left DLPFC; 20 ss | PANSS-NS/SANS: ↓ A;=S | TBS protocol showed higher reduction of NS. No significant difference between 10 Hz and 20 Hz groups. | |
| [51] | SZ/SAD=32 | 10 HzB; bilateral DLPFC; 15 ss | SANS: ↓ A;=S | SANS improvement was present up to 3 months follow-up. No change in PANSS-NS. | |
| [52] | SZ=117Â Â Â Â Â Â | 10 HzB; left DLPFC; 20 ss | PANSS/SANS: ↓ A;=S | Improvement of NS in TMS group persisted up to 24 weeks. No improvement in CGI-S. | |
| [60] | SZ=127 | 10 HzB; left DLPFC; 15 ss | PANSS-NS: ↓ A; ↓ S | No difference of improvement in the two groups for DS. Small improvement in Positive Symptoms at day 21. | |
| [53] | SZ/SAD=24 | 10 HzB; bilateral DLPFC; 15 ss | SANS: ↓ A;=S | Increased task related activation in TMS group in the right DLPFC and the right medial frontal gyrus. In the left posterior cingulate, decreased activation in the active and increased activation in the sham group. | |
| No difference in SANS score at the end of treatment between 2 groups. Improved SANS score in TMS after 8 weeks. | |||||
| [54] | SZ=47 | 10 HzB; left DLPFC; 20 ss | SANS: ↓ A; =S (over time) | ||
| Volume gains in the left hippocampal, parahippocampal and precuneal cortices and NS improvement in the active rTMS group showed a positive correlation. NS responders only in the active rTMS group. | |||||
| [61] | SZ=73 | 10 HzB; left DLPFC; 15 ss | PANSS-NS:=A;=S | ||
| Greater improvement in TMS group on CDSS. | |||||
| [66] | SZ=40 | TBSB; cerebellar vermal; 10 ss | PANSS-NS: ↓↓ A; ↓ S | ||
| Cognitive Dysfunction (CD) | [62] | SZ=16 | 20 HzB; left DLPFC; 10 ss | AVLT/TMT A, B:=A;=S | |
| [63] | SZ=17 | 10 HzB; left DLPFC; 10 ss | HVLT: ↑ A; =S (at 2 weeks) | There was no difference in the HVLT score between sham and active rTMS group at the end of treatment. | |
| [64] | SZ/SAD=15 | 10 HzB; bilateral PFC; 15 ss | TMT A, B:=A;=S | ||
| [55] | SZ=48 | 1 Hz/10 HzB; left DLPFC; 20 ss | WCST:=A;=S | ||
| [70] | SZ=25 | 10 HzB; left PMFG; 15 ss | 0,1,2-back:=A;=S | No activation differences (fMRI) over time in any sample. | |
| [72] | SZ=27 | 20 HzB; bilateral DLPFC; 20 ss | 3-back accuracy: ↑ A;=S | Improvement on 3-back accuracy (working memory performance) in TMS group (comparable to HC). No change in 1-back accuracy. | |
| [60] | SZ=127 | 10 HzB; left DLPFC; 15 ss | TMT A, B:=A;=S | ||
| No correlation with clinical improvement. | |||||
| [73] | SZ=36 | 10 HzB; left DLPFC; 10 ss | FART: ↑ A;=S | ||
| Improvement in Semantic Verbal Fluency up to 4 weeks. No improvement in AVLT, TMT A, B, WSCT. | |||||
| [51] | SZ/SAD=32 | 10 HzB; bilateral DLPFC; 15 ss | VFT: ↑ A (fluency); =S | ||
| [71] | SZ=156 | 10 HzB; left DLPFC | TMT A, B; WCST: ↑ A; ↑ S | ||
| In TMS group the decrease in the delta-band activity (associated with hypofrontality in SZ) originated in right prefrontal cortex and correlated with improvement in facial affect recognition. | |||||
| [74] | SZ=35 | 10 HzB; left DLPFC | FART: - |
Table 1: A summary of previous findings.
Acronyms?: AHRS=Auditory Hallucination Rating Scale; AVH-RAR=AVH related activation regions; AVLT=Auditory Verbal Learning Test; CDSS=Calgary Depression Scale for Schizophrenia; CGI-I=Clinical Global Impression-Improvement; CGI-S=Clinical Global Impression-Severity; COS=control site; DLPFC=dorsolateral prefrontal cortex; FART: Facial Affect Recognition Task; HCS=Hallucination Change Scale; HVLT=Hopkins Verbal Learning Test; LPA=Language Perception Area; ADRS=Montgomery- Asberg Depression Rating Scale; PANSS=Positive and Negative Symptoms Scale; PANSS-HS=PANSS Hallucination Subscale; PANSS-NS=PANSS negative symptom scores; PMFG=posterior middle frontal gyrus; PP=psychotic patients; SAH=Severity of Auditory Hallucinations; SANS=Scale for the Assessment of Negative Symptoms; SAPS=Scale for the Assessment of Positive Symptoms; SMG=supramarginal gyrus; SPT=Sylvian parietotemporal area; STG=superior temporal gyrus; VFT=Verbal Fluency Test; SZ=Schizophrenic Patients; TMT=Trail Making Test; TPC=temporo-parietal cortex; W=Wernicke; WCST=Wisconsin Card Sorting Test; A=only active; B=active and sham rTMS; C=priming and sham priming ; ↓/ ↑=significant reduction/increase;==no significant variation; ↓↓=significant higher reduction.
Results
The abovementioned search yielded 439 articles. After reviewing all articles, a total of 48 studies related to the treatment of auditory hallucinations, negative symptoms and cognitive dysfunction in schizophrenic patients using rTMS were selected (see Figure 1 for additional details). A summary of our findings is presented in Table 1.
Positive symptoms
We found many studies that targeted positive symptoms with rTMS treatment, and all of them focused on medicationresistant Auditory Verbal Hallucinations (AVH). In treating AVH, rTMS’s main target was the temporal cortex, based on the assumption that AVH manifestations are caused by hyperactivity of the temporal lobe [17]. The other main target was the left or bilateral Temporo-Parietal (TP) junction [18]. In these articles the primary outcome was assessed mostly using the Auditory Hallucination Rating Scale (AHRS) and the Hallucination Change Scale (HCS). Please see Table 1 for additional details.
We found that twenty-one studies used sham stimulation, another type of stimulation, or a control stimulation site for the active rTMS. Among these studies, six showed a significant clinical improvement in the active stimulation group when compared to the sham stimulation/control site stimulation, whereas fifteen did not.
Of the studies reporting a clinical improvement, one targeted the sylvian parietotemporal area, a sensorimotor language region that connects the sensory and motor speech systems [19]. This study employed 1 Hz rTMS and Theta Burst Stimulation (TBS), and found that both stimulations yielded a significant improvement in AVH scores, with no statistical difference between them. In both treatment groups, a reduction of blood flow in primary auditory cortex, left Broca's area, and cingulate gyrus was detected, and the reduction of primary auditory cortex blood flow was associated with an improvement in AVH scores [20]. In the other five studies the authors used 1 Hz rTMS on the left TP cortex, and the primary outcome (HCS/Severity of Auditory Hallucinations scale) was found to be improved in the active stimulation group after rTMS [21-25]. One study did not find an immediate response to TMS, but reported an improvement over time of the severity of auditory hallucination, of the Scale for the Assessment of Negative Symptoms (SANS) and Scale for the Assessment of Positive Symptoms (SAPS) scores in only the active group [22].
Fifteen studies failed to establish a statistical difference in AVH improvement between active rTMS and a sham/control stimulation, even though a reduction in AVH was commonly reported across treatment groups [26-40]. Ten of these studies targeted the left or bilateral TP region and employed 1 Hz rTMS, whereas the remaining five studies used slightly different stimulation parameters. Specifically, one study selectively stimulated left or right TP cortex using the centrooccipital cortex as control site and did not find any difference between stimulation sites, whereas another study used the vertex as control site for right and left temporal cortex rTMS [26,40]. A third study stimulated an individually selected language perception area, identified using a language auditory task during fMRI, while a fourth study targeted Wernicke’s region or Wernicke’s right homologous site [28,32]. Finally, in the last of these four studies rTMS targeted alternatively bilateral TP regions or Broca’s area with different stimulation patterns (1 Hz TP or 20 Hz TP or 20 Hz Broca’s area), although none of these stimulation paradigms was found to be more effective than sham [29]. Overall, although none of these fifteen studies reported a significant improvement of primary outcome measures, four found an improvement of secondary outcome scales, in particular in AVH severity [28,34,35,38].
We also found four studies that compared the effect of two different rTMS protocols on AVH, and all reported no statistically significant differences between stimulation protocols [41-44]. In two of these studies, 1 Hz rTMS was compared to TBS, both applied to the sylvian parietotemporal area, and the responders (n=9), identified as the patients with a 50% decrease in hallucination scores, differed from the nonresponders (n=15) because they had higher cerebral blood flow in left superior temporal gyrus [41,42]. In another study a 20- minutes, 1 Hz rTMS was compared with 13 trains of 20 Hz rTMS, with both protocols being performed for a week over cortical areas that showed higher bold activation while participants were experiencing AVH. In this study, both TMS groups experienced a decrease in AVH at the end of treatment, with no significant difference across groups [43]. In the last of these four studies, 40 participants with recent onset schizophrenia were treated with 1 Hz rTMS, following priming or sham priming stimulation [44]. Priming is a new technique consisting of high-frequency stimulation (6 Hz) followed by low frequency (1 Hz) rTMS. Past studies showed that pretreatment stimulation could augment the effect of the low frequency treatment, and that it was more effective than shampriming TMS of dorsolateral prefrontal cortex for depressive symptoms [45]. In contrast, in this last study both protocols had the same efficacy in treating AVH over time, although pretreatment priming was associated with a stronger reduction of AVH loudness during follow up [44].
Finally, one study targeting positive symptoms employed highfrequency (20 Hz) rTMS (rather than 1 Hz) to the left TP region and correlated anatomical features of skull and brain with response to rTMS in schizophrenia patients with AVH [46]. An association between temporal scalp-to-cortex distance and treatment efficacy was found, together with a correlation between treatment efficacy and grey matter density both in temporal and primary hand motor area.
Negative symptoms
Negative symptoms are frequently resistant to antipsychotic medications, which makes it extremely relevant to find treatment alternatives in schizophrenia [5]. The cortical region most commonly targeted by TMS is the dorsolateral prefrontal cortex (DLPFC), since neuroimaging studies have shown a correlation between reduction of cerebral blood flow in that area and higher prevalence of negative symptoms in schizophrenia [47]. Moreover, a PET study performed on schizophrenia patients with negative symptoms showed a decrease in glucose consumption in temporal and prefrontal cortices and a higher metabolic use of glucose in the cerebellar cortex [48].
We included seventeen studies that evaluated rTMS efficacy in treating negative symptoms in schizophrenic patients. Functional parameters typically chosen as primary outcome measure were the scale for the assessment of negative symptoms (SANS) and PANSS (Positive and Negative Symptoms Scale) negative symptoms subscale.
We found sixteen studies that employed rTMS active and sham stimulation on the left or bilateral DLPFC to treat the negative symptoms of patients with schizophrenia. Of these studies, ten reported that the group receiving active rTMS reported a significant improvement in negative symptoms compared to sham, whereas six did not. All studies with positive findings targeted the DLPFC with 10 Hz TMS, including three studies that compared the effect of different rTMS protocols on negative symptoms [49-58]. In one of them, a 10 Hz protocol was compared with 1 Hz rTMS in fifty-eight schizophrenic patients [55]. While 10 Hz stimulation significantly reduced SANS score, no difference was found between 1 Hz rTMS and sham. In the second study, ninety-three patients were divided in four groups and underwent 10 Hz, 20 Hz, TBS or sham stimulation respectively [50]. All patients treated with active rTMS had a reduction of SANS, PANSS general psychopathology and PANSS negative symptom subscale, although the TBS group experienced a larger decrease in symptoms compared to the 10 and 20 Hz rTMS groups. The last of these three studies compared the effect on negative symptoms of four different protocols: individualized alpha TMS, 3 Hz stimulation, 20 Hz stimulation and a sham protocol, and found that the alpha stimulation was superior to the other protocols in reducing PANSS negative scores [55]. While most studies reported an improvement in negative symptoms at the end of the treatment intervention with rTMS, some established a delayed effect on negative symptoms. For example, one study found that the active rTMS group did not experience an improvement in SANS scale up to 4 weeks, but at 8 weeks the score was significantly better than the sham group [54]. Another study established a significant improvement on negative symptoms after 6 weeks of active rTMS, which persisted up to 24 weeks of assessment, whereas no difference in the Clinical Global Impression (CGI) scale was found [52].
Six studies failed to find an improvement on negative symptoms in active rTMS compared to sham stimulation [59-64]. Two of those studies employed 20 Hz rTMS on bilateral DLPFC, whereas the remaining studies used 10 Hz rTMS on the left DLPFC, except for one that targeted bilateral DLPFC [59-64]. While most of these investigations were conducted on a relatively small group of patients (N<25), a couple had relatively large sample sizes. Specifically, in one study enrolling one hundred twenty-seven schizophrenia patients the authors found no difference between active and sham rTMS on negative symptoms, neither at day 21 nor 105, although a small, significant improvement in positive symptoms was found in the active rTMS group at day 21 [60]. The other study, which included seventy-three schizophrenia patients, found that left hippocampal, parahippocampal and precuneal cortices’ volume gain correlated with a decrease in negative symptoms only in the active rTMS group, although active stimulation was not better than sham stimulation in treating negative symptoms [61].
Outside of DLPFC, rTMS was used in one study to target the cerebellum, since cerebellar circuits have been implicated in the pathophysiology of the negative symptoms of schizophrenia [48,65]. This study compared TBS and sham stimulation of the cerebellar vermis in forty patients with schizophrenia and found that those receiving TBS showed a greater improvement both in negative and depressive symptoms [66].
Cognitive dysfunction
Cognitive impairments are core features of schizophrenia, which are associated with poor occupational and social functioning and predict long-term disability in those patients [67]. Working memory (WM) deficits in particular have been consistently associated with schizophrenia and are thought to depend on dysfunctions in DLPFC activity [68,69]. Thus, DLPFC has been the main target for rTMS protocols aimed at ameliorating cognitive functioning in schizophrenia.
Overall, eleven articles employed rTMS in schizophrenia to ameliorate cognitive dysfunctions, with the left DLPFC as the main target area. The most relevant neuropsychological tests and eventual improvement are reported in Table 1. Six studies found no significant differences between rTMS and sham stimulation in improving cognition, whereas five studies reported that the active stimulation was more beneficial [51,55,60,62-64,70-74]. Of these five studies, one showed that 20 Hz rTMS yielded an increase in 3-back accuracy in schizophrenia patients, which was found to be comparable to healthy subjects’ performances [72]. In another study, facial affect recognition was reported to be improved after stimulation of left DLPFC with 10 Hz rTMS, whereas the same stimulation paradigm improved verbal fluency and verbal memory in different groups of schizophrenic patients [51,63,73]. Finally, the last of these studies found a correlation between improvement in facial affect recognition and a reduction in delta-band activity during resting EEG, which is usually elevated in schizophrenia patients because of the decreased metabolism in their prefrontal lobes [74]. Despite these encouraging findings, it is important to point out that the six studies with negative results employed similar rTMS paradigms, and that two of those studies were conducted on large sample sizes of schizophrenia patients.
Conclusion
In this article we reviewed studies employing rTMS as a treatment tool in schizophrenia patients. Positive symptoms, negative symptoms and cognitive dysfunctions were primarily targeted, and the main findings from these studies were presented above. In what follows, we will briefly discuss how these findings may contribute to develop more effective TMSbased therapeutic interventions in schizophrenia.
TMS and positive symptoms: Among positive symptoms, medication-resistant auditory verbal hallucinations were most commonly investigated, with a rTMS protocol targeting the TP cortex at 1 Hz. Several recently published reviews and metaanalyses have found an improvement after rTMS treatment, although results were inconsistent across studies, to the extent that one of these reviews reported negative findings in eight out of eighteen studies, whereas another one clearly stated that there was a large heterogeneity across studies [75-77]. Here we found that the majority of these studies failed to find a difference between rTMS and sham stimulation, as well as between two different rTMS protocols. Several factors likely account for this outcome. First, we applied stringent criteria to assess positive and negative findings, by identifying as positive only an improvement in the scale chosen as primary outcome measure. Second, previous reviews cited several studies with fairly small sample size, whereas we selected only those with at least 15 participants. Third, recent articles showing negative results were not included in those reviews. Notably, although there is no strong evidence that rTMS is more effective than sham stimulation, factors like scalp to cortex distance and grey matter density, which are known to influence the response to TMS, have not been accounted for in these treatment studies [46]. It was also recently shown that regional blood flow is associated to response to active stimulation, which implies that a pretreatment MRI scans may help identify those patients that would benefit from rTMS [41]. Therefore, future treatment trials that account for these variables, and possibly optimize stimulation parameters accordingly, should increase the beneficial effects of rTMS on the positive symptoms of schizophrenia patients.
TMS and negative symptoms: TMS treatment studies of refractory negative symptoms targeted primarily DLPFC with 10 Hz rTMS. One review found an improvement in negative symptoms in five studies out of ten [77]. Furthermore, a metaanalysis performed on seven studies showed no improvement, whereas another review found a modest improvement in negative symptoms, which is in line with the results of our study [76-78]. In our review, we reported that most of these studies found that active stimulation was more beneficial than sham, although with some variability. Moreover, one study comparing three different active stimulation protocols found TBS to be superior to 10 and 20 Hz rTMS [50]. Thus, various rTMS paradigms have shown to ameliorate negative symptoms in schizophrenia, and TBS of DLPFC appears the most promising pattern of stimulation to date. However, future studies in larger groups of patients, including investigations comparing the effectiveness of different rTMS paradigms in double blind, randomized clinical trials, are needed to fully establish the effectiveness of these treatment interventions.
TMS and cognitive dysfunctions: These TMS studies mostly targeted left DLPFC using a high-frequency stimulation of 10 Hz. Although we found that studies involving a large group of patients report negative findings, one study that targeted bilateral DLPFC using 20 Hz showed a significant improvement in working memory in schizophrenia patients [72]. We believe these findings are encouraging, since another meta-analysis found a positive effect of rTMS on working memory, and a second review found an indication of rTMS beneficial effect on visual, verbal and working memory [79,80]. Nonetheless, future studies employing large samples of participants are required to evaluate if this effect is actually replicable or just restricted to a limited group of patients. Furthermore, given the small number (N=11) of studies conducted so far, future work will help identify which aspects of cognitive functioning may be more responsive to rTMS treatment interventions.
References
- Insel TR. Rethinking schizophrenia. Nature. 2010;468(7321):187-93.
- Saha S, Chant D, Welham J, et al. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2(5):e141.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Publishing. Arlington, US. 2013.
- Biedermann F, Fleischhacker WW. Emerging drugs for schizophrenia. Expert Opin Emerg Drugs. 2011;16(4):271-82.
- Leucht S, Corves C, Arbter D, et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. The Lancet. 2009;373(9657):31-41.
- Kaskie RE, Ferrarelli F. Investigating the neurobiology of schizophrenia and other major psychiatric disorders with Transcranial Magnetic Stimulation. Schizophr Res. 2017.
- Conn PJ, Tamminga C, Schoepp DD, et al. Schizophrenia: Moving beyond monoamine antagonists. Mol Interv. 2008;8(2):99-107.
- Aleman A. Use of repetitive transcranial magnetic stimulation for treatment in psychiatry. Clin Psychopharmacol Neurosci. 2013;11(2):53.
- Wassermann, Eric M. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation. Electroencephalogr Clin Neurophysiol /Evoked Potentials Section. 1996;108(1):1-16.
- Pascual-Leone A, Amedi A, Fregni F, et al. The plastic human brain cortex. Annu Rev Neurosci. 2005;28:377-401.
- Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. 2003;148(1):1-16.
- Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117(12):2584-96.
- Keck ME, Welt T, Müller MB, et al. Repetitive transcranial magnetic stimulation increases the release of dopamine in the mesolimbic and mesostriatal system. Neuropharmacol. 2003;43(1):101-9.
- Strafella AP, Paus T, Barrett J, et al. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21(15):RC157.
- Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PloS One. 2009;21:e6725.
- Cristancho MA, Cristancho P, O?reardon JP. Other therapeutic psychiatric uses of superficial brain stimulation. Handb Clin Neurol. 2013;116:415-22.
- Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage. 2011;57(3):1221-33.
- Bais L, Liemburg E, Vercammen A, et al. Effects of low frequency rTMS treatment on brain networks for inner speech in patients with schizophrenia and auditory verbal hallucinations. Prog Neuropsychopharmacol Biol Psychiatry. 2017.
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature reviews. Neurosci. 2007;8(5):393.
- Kindler J, Homan P, Jann K, et al. Reduced neuronal activity in language-related regions after transcranial magnetic stimulation therapy for auditory verbal hallucinations. Biol Psychiatry. 2013;73(6):518-24.
- Hoffman RE, Hawkins KA, Gueorguieva R, et al. Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch Gen Psychiatry. 2003;60(1):49-56.
- Chibbaro G, Daniele M, Alagona G, et al. Repetitive transcranial magnetic stimulation in schizophrenic patients reporting auditory hallucinations. Neurosci Lett. 2005;383:54-57.
- Hoffman RE, Gueorguieva R, Hawkins KA, et al. Temporoparietal transcranial magnetic stimulation for auditory hallucinations: safety, efficacy and moderators in a fifty patient sample. Biol Psychiatry. 2005;58:97-104.
- Brunelin J, Poulet E, Bediou B, et al. Low frequency repetitive transcranial magnetic stimulation improves source monitoring deficit in hallucinating patients with schizophrenia. Schizophr Res 2006;81:41-5.
- Bagati D, Nizamie SH, Prakash R. Effect of augmentatory repetitive transcranial magnetic stimulation on auditory hallucinations in schizophrenia: randomized controlled study. Australian New Zealand J Psychiatry. 2009;43(4):386-92.
- van Lutterveld R, Koops S, Schutter DJ, et al. The effect of rTMS on auditory hallucinations: clues from an EEG-rTMS study. Schizophr Res. 2012;137:174-9.
- Bais L, Vercammen A, Stewart R, et al. Short and long term effects of left and bilateral repetitive transcranial magnetic stimulation in schizophrenia patients with auditory verbal hallucinations: a randomized controlled trial. PloS One. 2014;9:e108828.
- Hoffman RE, Wu K, Pittman B, et al. Transcranial magnetic stimulation of Wernicke?s and Right homologous sites to curtail ?voices?: a randomized trial. Biol Psychiatry. 2013;73:1008-14.
- Kim EJ, Yeo S, Hwang I, et al. Bilateral repetitive transcranial magnetic stimulation for auditory hallucinations in patients with schizophrenia: a randomized controlled, cross-over study. Clin Psychopharmacol Neurosci. 2014;12(3):222.
- Koops S, Dellen EV, Schutte MJ, et al. (2015) Theta burst transcranial magnetic stimulation for auditory verbal hallucinations: negative findings from a double-blind-randomized trial. Schizophr Bull. 2015;42(1):250-7.
- Kimura H, Kanahara N, Takase M, et al. A randomized, sham-controlled study of high frequency rTMS for auditory hallucination in schizophrenia. Psychiatry Res. 2016;241:190-4.
- Paillère-Martinot ML, Galinowski A, Plaze M, et al. Active and placebo transcranial magnetic stimulation effects on external and internal auditory hallucinations of schizophrenia. Acta Psychiatr Scand. 2017;135(3):228-38.
- McIntosh AM, Semple D, Tasker K, et al. Transcranial magnetic stimulation for auditory hallucinations in schizophrenia. Psychiatry Res. 2004;127(1-2):9-17.
- Lee SH, Kim W, Chung YC, et al. A double blind study showing that two weeks of daily repetitive TMS over the left or right temporoparietal cortex reduces symptoms in patients with schizophrenia who are having treatment-refractory auditory hallucinations. Neurosci Lett. 2005;376(3):177-81.
- Vercammen A, Knegtering H, Bruggeman R, et al. Effects of bilateral repetitive transcranial magnetic stimulation on treatment resistant auditory?verbal hallucinations in schizophrenia: a randomized controlled trial. Schizophr Res. 2009;114:172-9.
- Vercammen A, Knegtering H, Liemburg EJ, et al. Functional connectivity of the temporo-parietal region in schizophrenia: effects of rTMS treatment of auditory hallucinations. J Psychiatric Res. 2010;44:725-31.
- Slotema CW, Blom JD, de Weijer AD, et al. Can low-frequency repetitive transcranial magnetic stimulation really relieve medication-resistant auditory verbal hallucinations? Negative results from a large randomized controlled trial. Biol Psychiatry. 2010;69(5):450-6.
- de Jesus DR, Gil A, Barbosa L, et al. A pilot double-blind sham-controlled trial of repetitive transcranial magnetic stimulation for patients with refractory schizophrenia treated with clozapine. Psychiatry Res. 2011;188:203-7.
- Fitzgerald PB, Benitez J, Daskalakis JZ, et al. A double-blind sham-controlled trial of repetitive transcranial magnetic stimulation in the treatment of refractory auditory hallucinations. J Clin Psychopharmacol. 2005;25(4):358-62.
- Loo CK, Sainsbury K, Mitchell P, et al. A sham-controlled trial of left and right temporal rTMS for the treatment of auditory hallucinations. Psychol Med. 2010;40:541-6.
- Homan P, Kindler J, Hauf M, et al. Cerebral blood flow identifies responders to transcranial magnetic stimulation in auditory verbal hallucinations. Transl Psychiatry. 2012;2(11):e189.
- Kindler J, Homan P, Flury R, et al. Theta burst transcranial magnetic stimulation for the treatment of auditory verbal hallucinations: results of a randomized controlled study. Psychiatry Res 2013;209:114-7.
- De Weijer AD, Sommer IE, Meijering AL, et al. High frequency rTMS; a more effective treatment for auditory verbal hallucinations? Psychiatry Res: Neuroimaging. 2014;224:204-10.
- Ray P, Sinha VK, Tikka SK. Adjuvant low-frequency rTMS in treating auditory hallucinations in recent-onset schizophrenia: a randomized controlled study investigating the effect of high-frequency priming stimulation. Ann Gen Psychiatry. 2015;14:8.
- Iyer MB, Schleper N, Wassermann EM. Priming stimulation enhances the depressant effect of low-frequency repetitive transcranial magnetic stimulation. J Neurosci. 2013;23(34):10867-72.
- Nathou C, Simon G, Dollfus S, et al. Cortical anatomical variations and efficacy of rTMS in the treatment of auditory hallucinations. Brain Stimul. 2015;8(6):1162-7.
- Sabri O, Erkwoh R, Schreckenberger M, et al. Regional cerebral blood flow and negative/positive symptoms in 24 drug-naive schizophrenics. J Nucl Med. 1997;38(2):181.
- Potkin SG, Alva G, Fleming K, et al. A PET study of the pathophysiology of negative symptoms in schizophrenia. Am J Psychiatry. 2002;159(2):227-37.
- Prikryl R, Ustohal L, Kucerova HP, et al. A detailed analysis of the effect of repetitive transcranial magnetic stimulation on negative symptoms of schizophrenia: a double-blind trial. Schizophr Res. 2013;149(1-3):167-73.
- Zhao S, Kong J, Li S, et al. Randomized controlled trial of four protocols of repetitive transcranial magnetic stimulation for treating the negative symptoms of schizophrenia. Shanghai Arch Psychiatry. 2014;26(1):15-21.
- Dlabac-de Lange JJ, Bais L, van Es FD, et al. Efficacy of bilateral repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: results of a multicenter double-blind randomized controlled trial. Psychol Med. 2015;45(6):1263-75.
- Quan WX, Zhu XL, Qiao H, et al. The effects of high-frequency repetitive transcranial magnetic stimulation (rTMS) on negative symptoms of schizophrenia and the follow-up study. Neurosci Lett. 2015;584:197-201.
- Dlabac-de Lange JJ, Liemburg EJ, Bais L, et al. Effect of rTMS on brain activation in schizophrenia with negative symptoms: A proof-of-principle study. Schizophr Res. 2015;168:475-82.
- Li Z, Yin M, Lyu XL, et al. Delayed effect of repetitive transcranial magnetic stimulation (rTMS) on negative symptoms of schizophrenia: findings from a randomized controlled trial. Psychiatry Res. 2016;240:333-5.
- Schneider AL, Schneider TL, Stark H. Repetitive transcranial magnetic stimulation (rTMS) as an augmentation treatment for the negative symptoms of schizophrenia: a 4-week randomized placebo controlled study. Brain Stimul. 2016;1:106-11.
- Jin Y, Potkin SG, Kemp AS, et al. Therapeutic effects of individualized alpha frequency transcranial magnetic stimulation (aTMS) on the negative symptoms of schizophrenia. Schizophr Bull. 2006;32(3):556-61.
- Prikryl R, Kasparek T, Skotakova S, et al. Treatment of negative symptoms of schizophrenia using repetitive transcranial magnetic stimulation in a double-blind, randomized controlled study. Schizophr Res. 2007;95(1-3):151-7.
- Hajak G, Marienhagen J, Langguth B, et al. High-frequency repetitive transcranial magnetic stimulation in schizophrenia: a combined treatment and neuroimaging study. Psychol Med. 2004;34(7):1157-63.
- Barr MS, Farzan F, Tran LC, et al. A randomized controlled trial of sequentially bilateral prefrontal cortex repetitive transcranial magnetic stimulation in the treatment of negative symptoms in schizophrenia. Brain stimul. 2012;5(3):337-46.
- Wobrock T, Guse B, Cordes J, et al. Left prefrontal high-frequency repetitive transcranial magnetic stimulation for the treatment of schizophrenia with predominant negative symptoms: a sham-controlled, randomized multicenter trial. Biol Psychiatry. 2012;77(11):979-88.
- Hasan A, Wobrock T, Guse B, et al. Structural brain changes are associated with response of negative symptoms to prefrontal repetitive transcranial magnetic stimulation in patients with schizophrenia. Mol Psychiatry. 2017;22(6):857-64.
- Novak T, Horacek J, Mohr P, et al. The double-blind sham-controlled study of high-frequency rTMS (20Hz) for negative symptoms in schizophrenia: negative results. Neuroendocrinol Lett. 2006;27(1-2):209-13.
- Mogg A, Purvis R, Eranti S, et al. Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: a randomized controlled pilot study. Schizophr Res. 2007;93(1-3):221-8.
- Fitzgerald PB, Herring S, Hoy K, et al. A study of the effectiveness of bilateral transcranial magnetic stimulation in the treatment of the negative symptoms of schizophrenia. Brain Stimul. 2008;1:27-32.
- Parker KL, Narayanan NS, Andreasen NC. The therapeutic potential of the cerebellum in schizophrenia. Front Syst Neurosci. 2014;8:163.
- Garg S, Sinha VK, Tikka SK, et al. The efficacy of cerebellar vermal deep high frequency (theta range) repetitive transcranial magnetic stimulation (rTMS) in schizophrenia: A randomized rater blind-sham controlled study. Psychiatry Res. 2016;243:413-20.
- Green MF, Kern RS, Braff DL, et al. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the ?right stuff?? Schizophr Bull. 2000;26(1):119-136.
- Forbes NF, Carrick LA, McIntosh AM, et al. Working memory in schizophrenia: a meta-analysis. Psychol Med. 2009;39(6):889-905.
- Barch DM, Carter CS, Braver TS, et al. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58(3):280-8.
- Guse B, Falkai P, Gruber O, et al. The effect of long-term high frequency repetitive transcranial magnetic stimulation on working memory in schizophrenia and healthy controls?a randomized placebo-controlled, double-blind fMRI study. Behavioural Brain Res. 2013;237:300-7.
- Hasan A, Guse B, Cordes J, et al. Cognitive effects of high-frequency rTMS in schizophrenia patients with predominant negative symptoms: results from a multicenter randomized sham-controlled trial. Schizophr Bull. 2016;42(3):608-18.
- Barr MS, Farzan F, Rajji TK, et al. Can repetitive magnetic stimulation improve cognition in schizophrenia? Pilot data from a randomized controlled trial. Biol Psychiatry. 2013;73(6):510-7.
- Wölwer W, Lowe A, Brinkmeyer J, et al. Repetitive transcranial magnetic stimulation (rTMS) improves facial affect recognition in schizophrenia. Brain Stimul. 2014;7:559-63.
- Kamp D, Brinkmeyer J, Agelink MW, et al. High frequency repetitive transcranial magnetic stimulation (rTMS) reduces EEG-hypofrontality in patients with schizophrenia. Psychiatry Res. 2015;236:199-201.b.
- Otani VH, Shiozawa P, Cordeiro Q, et al. A systematic review and meta-analysis of the use of repetitive transcranial magnetic stimulation for auditory hallucinations treatment in refractory schizophrenic patients. Int J Psychiatry Clin Pract. 2015;19(4): 228-32.
- He H, Lu J, Yang L, et al. Repetitive transcranial magnetic stimulation for treating the symptoms of schizophrenia: A PRISMA compliant meta-analysis. Clin Neurophysiol. 2017;128(5):716-24.
- Cole JC, Bernacki CG, Helmer A, et al. Efficac