Research Article - Biomedical Research (2019) Volume 30, Issue 6
Toxicological perspective of cadmium on estuarine edible crab Scylla serrata regarding ovarian maturation.
Revathy R1, Langeswaran VK2*, Balasubramanian MP11Department of Pharmacology and Environmental Toxicology, University of Madras, Taramani, Chennai, Tamil Nadu, India
2Department of Bioinformatics, Cancer Genetics and Molecular Biology Laboratory, Science Campus, Alagappa University, Karaikudi, Tamil Nadu, India
- *Corresponding Author:
- Langeswaran VK
Assistant ProfessorDepartment of Bioinformatics
Cancer Genetics and Molecular Biology Laboratory
Science Campus,Alagappa University
Karaikudi-630003, Tamil Nadu
India
Accepted Date: October 04, 2019
Abstract
Background: Cadmium is more toxic to animals and humans even at low concentration than utmost
toxic metals, and naturally they are non-degradable can disorder biological systems. Industries and
factories cadmium salts liquefy in environmental waste water and finally interrupt the marine living
systems.
Aim: The present study aim is to investigate the impact of cadmium in the estuarine edible crab
Scylla serrata regarding some biochemical constituents.
Methodology: Crabs were divided into two groups whereas crabs belonging to group I were treated
as control (cadmium free-seawater). Crabs belonging to group II were exposed to cadmium at 20
mg/L concentration. The transaminase activity and estimation antioxidants enzymes have been
analyzed in the ovary, hepatopancreas, muscle and haemolymph of edible crab Scylla serrate during
several periods of the ovarian maturation.
Interpretation: Notable alterations in the activities of biochemical constituents and reproductively
active aquatic animal’sphysiological occupations were totally uncharacterized due to heavy metal
pollution and finally injury progression of reproduction. The outcomes exposed that, amplified
levels of transaminase and contractions activity of antioxidants in the cadmium reared crabs when
compared to the control. The observed results of this experiment were tested statistically
Keywords
Cd, Cadmium, Scylla Serrata, Heavy metal, Edible Crab, Environmental safety.
Introduction
Environmental safety and pollutants is being debatable problems worldwide recently and based on this strategy there is a necessity to focus research on these aspects owing to the modernization and urbanization. Currently it is an active field of research worldwide by the scientists engaged in search and studies on the adverse effects of the hazardous pollutants in the ecosystems [1]. Pollutants play a significant role in the areas related to environmental toxicology and have a great attention for their devastating toxications in living biological systems. Pollutants are of many types based on their properties, exudates from types of industries and competence to produce undesirable toxicological effects in the living organisms. It is well recognized that human beings are not exemption from the pollutants harm and they indeed to affect by the pollutants either directly or indirectly [2]. The natural toxicity of a chemical can be transformed by the presence of another chemical. It may upsurge the toxicity namely synergism or it may diminish the toxic effect called as antagonism or it be neutral or having no interactions. The scattering of chemical and their metabolites throughout organism’s lifespan is estimated by the bioaccumulation and biochemical biomarkers of toxicant exposure. The exposure of toxicant and their effects in organisms were estimated through biochemical fluctuations are the first measurable and qualitative responses to environmental vicissitudes [3].
Cadmium occur in both terrestrial and aquatic surface and small quantity of cadmium existence deliver harmful effects on biological organization. Ingestion or intake of cadmium contaminated marine flesh like shellfish or green vegetables through diet is the major pathway for cadmium toxicity in humans [4]. Applying sewage sludge to soils (cadmium rich ‘biosolids’) used for crops destined for human consumption, or of using cadmium enriched phosphate fertilizer leads to accumulation of cadmium and subsequent hazards to human health. Every year the concentrations of cadmium is being biomagnified more than 12 times within food web of two trophic links. Cadmium is highly sensitive to aquatic wildlife and to other fish varieties being particularly susceptible to low quantity of this heavy metal [5]. Generally, crustaceans have been found to be useful as indicators and integrators of certain contaminants due to their wide geographic distribution, dominant presence in coastal and estuarine communities, bottom-dwelling habitats, ability to respond to environmental pollutants [6]. Hence, it is of interest to investigate the impact of cadmium in the estuarine edible crab Scylla serrata regarding some biochemical constituents (Graph 1).
Materials and Methods
Experimental animal
Scylla belongs to the family portunidae also known as mud crabs. Regarding the biology of crustaceans several research works has been carried out [7,8]. However, little information is available on the effect of pollutants on decapod crustaceans. Hence, it is proposed to investigate the effect of cadmium on the edible estuarine crab, Scylla serrata with reference to the ovarian maturation.
Collection of the animals
Female Scylla serrata were collected from the Pulicat Lake near Chennai, Tamilnadu, India. They were acclimatized to the laboratory condition (28°-2°C) in large glass aquaria for a week. The volume of water was adjusted so that the individuals were just submerged. They were fed with flesh of fresh prawns. The water was changed daily and the crabs were acclimated to the research laboratory conditions.
Toxicant
Cadmium is a non-essential metal [9] and a non-degradable pollutant. It is a common impurity in zinc, and it is most often isolated during the production of zinc. It is highly toxic at relatively low concentrations, readily accumulates in tissues and can adversely affect the organisms. It has a long biological half-life [10]. It is found in small quantities in natural waters and is common in the effluents of mining, electroplating and paint industries.
Experimental design
After acclimatization to the laboratory condition, crabs were divided into two groups. Crabs belonging to group I were reared in cadmium free-seawater and treated as control. Crabs belonging to group II were exposed to cadmium at 20 mg/L concentration. The treatments were continued up to 96 h. After exposure to cadmium, the ovarian stages were classified based on the criteria explained [11].
Stage I – Immature, prepubertal and reproductively inactive ovary; white and thread like in appearance.
Stage II – Reproductively active, showing peripheral undulations for the formation of ovarioles and white in colour.
Stage III – Ovariole formation and oogonial proliferation completed. The ovary is white in colour, opaque and thicker than the previous stage.
Stage IV – Beginning of vitellogenesis and the ovary acquires colouration from pale to deep yellow.
Stage V – Bright orange – coloured ovary with vitellogenesis at its peak lipid yolk deposition completed.
Collection of samples
Ovary, muscle and hepatopancreas were separated from the exposed and control crabs. The haemolymph was collected after prechilling the animals for 5 min [12]. This procedure prevents alterations and clumping of haemocytes and melanization. The haemolymph was drained directly into prechilled centrifuge tube from the cut end of propodus or dactylus of an appendage. The whole haemolymph including the haemocytes was used for biochemical analysis.
Biochemical estimation
Estimation of transaminase activity: In the present investigation, the estimation of Transaminase systems such as Alanine Transaminase (ALT) and Aspartate Transaminase (AST) activity were determined [13].
Assay of antioxidants: The activity of superoxide dismutase was determined by the method [14], CAT was assayed by the method [15]. GPx activity was assessed by the method [16], Ascorbic acid was estimated by the method [17], The level of vitamin E was estimated by the method [18].
Statistical analysis
The data were expressed as mean ± SD. Results were analyzed statistically by student T-test using SPSS software student’s version.
Results and Discussion
Aspartate transaminase
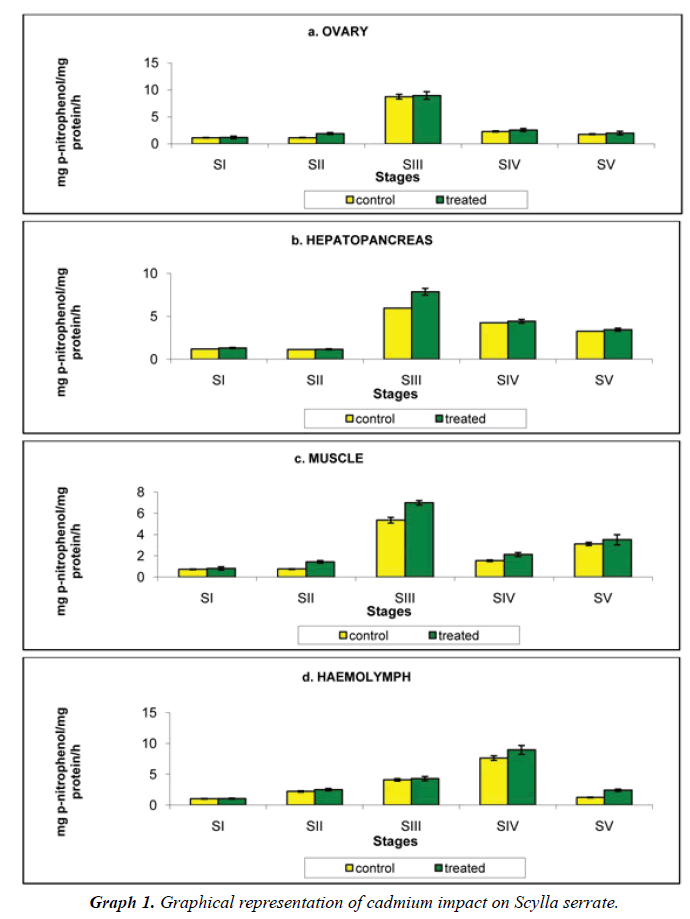
Ovary: Aspartate transaminase detected in the ovary of crabs reared in Cadmium free medium was 1.123, 0.127, 8.723, 2.274 and 1.771 mg pyruvate/mg protein/h increased to 1.168, 1.890, 8.951, 2.547 and 1.969 mg p-nitrophenol/ mg protein/h at stages I to V respectively, when reared in cadmium medium (Figure 1a).
Hepatopancreas: Level of Aspartate transaminase was the highest in the hepatopancreas among the three tissues analysed in the present investigation. The hepatopancreatic Aspartate transaminase activity of crabs reared in cadmium free medium was 1.188, 1.123, 5.957, 4.238 and 3.258 mg pyruvate/mg protein/h, increased to 1.309, 1.159, 7.858, 4.416 and 3.444 mg pyruvate/mg protein/h at stages I to V respectively, when reared at cadmium medium (Figure 1b).
Muscle: Level of muscle Aspartate transaminase of crabs reared in cadmium free medium was 0.725, 0.752, 5.348, 1.534 and 3.114 mg pyruvate/mg protein/h. when crabs reared in 20 mg/L concentration of cadmium medium, the level of muscle Aspartate transaminase increased to 0.810, 1.429, 6.990, 2.114 and 3.509 mg pyruvate/mg protein/h (Figure 1c).
Haemolymph: The circulating fluid haemolymph has a moderate level of aspartate transaminase, was 1.019, 2.208, 4.089, 7.615 and 1.233 mg pyruvate/mg protein/h in the control animals on exposure to 20 mg/L of cadmium concentration, it increased to 1.047, 2.504, 4.293, 8.952 and 2.405 mg pyruvate/mg protein/h. at stages I to V respectively (Table 1 and Figure 1d).
| Ovarian Developmental stages | Â Ovary | Hepatopancreas | Â Muscle | Haemolymph | |
|---|---|---|---|---|---|
| Stage I | Control | 1.123 ± 0.013 | 1.188 ± 0.067 | 0.725 ± 0.010 | 1.019 ± 0.003 |
| Treated | 1.168 ± 0.251** | 1.309 ± 0.250** | 0.810 ± 0.152*** | 1.047 ± 0.120** | |
| Stage II | Control | 1.127 ± 0.012 | 1.123 ± 0.012 | 0.752 ± 0.006 | 2.208 ± 0.006 |
| Treated | 1.890 ± 0.180** | 1.159 ± 0.210** | 1.429 ± 0.124** | 2.504 ± 0.140** | |
| Stage III | Control | 8.723 ± 0.197 | 5.957 ± 0.040 | 5.348 ± 0.020 | 4.089 ± 0.020 |
| Treated | 8.951 ± 0.697* | 7.858 ± 0.844* | 6.990 ± 0.213*** | 4.293 ± 0.213* | |
| Stage IV | Control | 2.274 ± 0.027 | 4.238 ± 0.077 | 1.534 ± 0.326 | 7.615 ± 0.326 |
| Treated | 2.547 ± 0.289*** | 4.416 ± 0.314* | 2.114 ± 0.191*** | 8.952 ± 0.540*** | |
| Stage V | Control | 1.771 ± 0.008 | 3.258 ± 0.053 | 3.114 ± 0.119 | 1.233 ± 0.119 |
| Treated | 1.969 ± 0.333*** | 3.444 ± 0.202*** | 3.509 ± 0.484*** | 2.405 ± 0.250*** | |
Values were expressed as mean ± SD of 6 observations. Asterisks indicate values that are significantly different from control, *p<0.5, *p<0.01, *p<0.001.
Table 1: Variation in the Aspartate transaminase activity of ovary, hepatopancreas, muscle and haemolymph in the control and cadmium chloride exposed Scylla serrata in different ovarian stages. The results were expressed in mg pyruvate/mg protein/h.
Alkaline transaminase
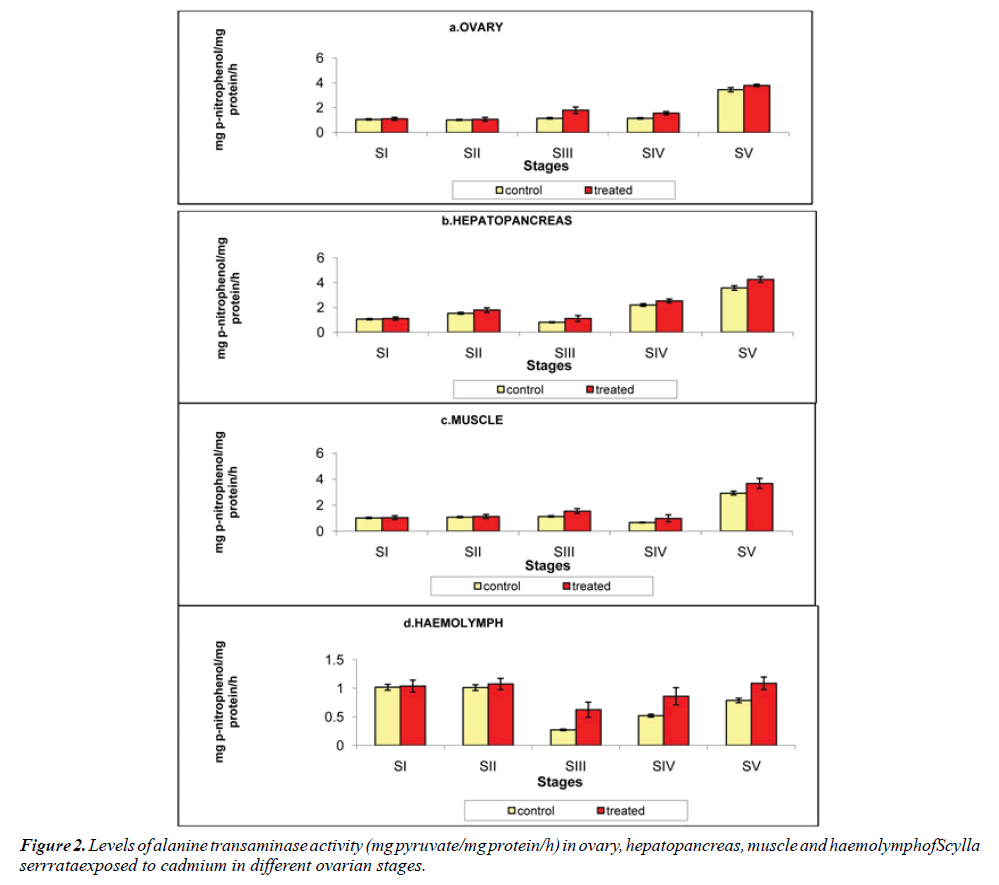
Ovary: The ovarian alanine transaminase level was 1.048, 1.008, 1.141, 1.132 and 3.442 mg pyruvate/mg protein/h at stages I to V respectively in crabs reared in chemical-free medium. On exposure to cadmium, it increased to 1.094, 1.056, 1.787, 1.552 and 3.778 mg pyruvate/mg protein/h at stages I to V respectively (Figure 2a).
Hepatopancreas: As recorded for the aspartate transaminase activity, the alanine transaminase activity was also to its maximum in the hepatopancreas. It was analyzed that the alanine transaminase activity was 1.051, 1.531, 0.802, 2.196 and 3.568 mg pyruvate/mg protein/h in control crabs increased to 1.016, 1.782, 1.111, 2.526 and 4.246 mg pyruvate/mg protein/h in cadmium exposed crabs, at stage I to V respectively (Figure 2b).
Muscle: In the muscle of the control animals the level of alanine transaminase activity was 1.018, 1.087, 1.132, 0.665 and 2.922 mg pyruvate/mg protein/h. on exposure to cadmium, the alanine transaminase activity level was increased to 1.039, 1.128, 1.547, 0.981 and 3.672 mg pyruvate/mg protein/h at stages I to V respectively (Figure 2c).
Haemolymph: Influence of cadmium on the haemolymph alanine transaminase activity of Scylla serrata was 1.018, 1.012, 0.273, 0.521 and 0.787 mg pyruvate/mg protein/h at stages I to V respectively in the cadmium-free medium and it was increased to 1.039, 1.074, 0.625, 0.860 and 1.087 mg pyruvate/mg protein/h at stages I to V respectively, when reared in cadmium medium (Table 2 and Figure 2d).
| Ovarian Developmental stages | Â Ovary | Hepatopancreas | Â Muscle | Haemolymph | |
|---|---|---|---|---|---|
| Stage I | Control | 1.048 ± 0.044 | 1.051 ± 0.008 | 1.018 ± 0.004 | 1.018 ± 0.004 |
| Treated | 1.094 ± 0.120** | 1.106 ± 0.131*** | 1.039 ± 0.145*** | 1.039 ± 0.105*** | |
| Stage II | Control | 1.008 ± 0.004 | 1.531 ± 0.007 | 1.087 ± 0.007 | 1.012 ± 0.003 |
| Treated | 1.056 ± 0.160*** | 1.782 ± 0.192*** | 1.128 ± 0.158*** | 1.074 ± 0.100*** | |
| Stage III | Control | 1.141 ± 0.032 | 0.802 ± 0.010 | 1.132 ± 0.034 | 0.273 ± 0.029 |
| Treated | 1.787 ± 0.262*** | 1.111 ± 0.238** | 1.547 ± 0.185*** | 0.625 ± 0.133*** | |
| Stage IV | Control | 1.132 ± 0.029 | 2.196 ± 0.039 | 0.665 ± 0.333 | 0.521 ± 0.014 |
| Treated | 1.552 ± 127*** | 2.526 ± 0.156*** | 0.981 ± 0.269*** | 0.860 ± 0.151*** | |
| Stage V | Control | 3.442 ± 0.058 | 3.568 ± 0.075 | 2.922 ± 0.082 | 0.787 ± 0.024 |
| Treated | 3.788 ± 0.92*** | 4.246 ± 0.232*** | 3.672 ± 0.383*** | 1.087 ± 0.110*** | |
Values were expressed as mean ± SD of 6 observations. Asterisks indicate values that are significantly different from control, *p<0.01, *p<0.001.
Table 6: Variation in the Alanine transaminase activity of ovary, hepatopancreas, muscle and haemolymph in the control and cadmium chloride exposed Scylla serrata in different ovarian stages. The results were expressed in mg pyruvate/mg protein/h.
Enzymic antioxidants
Superoxide dismutase (SOD): The SOD is a collection of metalloenzymes that catalyze the conversion of reactive superoxide anions (O2) to yield hydrogen peroxide (H2O2). SOD is a tremendous antioxidant enzymes and their occurrence is found in all aerobic organisms studied [19] (Table 3).
| Ovarian Developmental stages | Â Ovary | Hepatopancreas | Â Muscle | Haemolymph | |
|---|---|---|---|---|---|
| Stage I | Control | 0.36 ± 0.05 | 0.73 ± 0.06 | 0.65 ± 0.05 | 0.37 ± 0.008 |
| Treated | 0.23 ± 0.03** | 0.65 ± 0.04* | 0.55 ± 0.03** | 0.33 ± 0.006** | |
| Stage II | Control | 0.22 ± 0.04 | 0.58 ± 0.05 | 0.42 ± 0.06 | 0.29 ± 0.006 |
| Treated | 0.19 ± 0.02* | 0.44 ± 0.03** | 0.39 ± 0.02** | 0.26 ± 0.004* | |
| Stage III | Control | 0.43 ± 0.05 | 0.87 ± 0.05 | 0.78 ± 0.04 | 0.49 ± 0.007 |
| Treated | 0.36 ± 0.03** | 0.76 ± 0.03** | 0.64 ± 0.03** | 0.29 ± 0.005** | |
| Stage IV | Control | 0.55 ± 0.03 | 0.92 ± 0.06 | 0.86 ± 0.04 | 0.62 ± 0.006 |
| Treated | 0.48 ± 0.01** | 0.82 ± 0.04** | 0.77 ± 0.02** | 0.59 ± 0.005** | |
| Stage V | Control | 0.28 ± 0.03 | 0.66 ± 0.05 | 0.53 ± 0.03 | 0.53 ± 0.005 |
| Treated | 0.26 ± 0.01** | 0.54 ± 0.03** | 0.48 ± 0.02** | 0.43 ± 0.003** | |
Values were expressed as mean ± SD of 6 observations. Asterisks indicate values that are significantly different from control, *p<0.5, *p<0.01.
Table 3: Variation in the Superoxide dismutase activity of ovary, hepatopancreas, muscle and haemolymph in the control and cadmium chloride exposed Scylla serrata in different ovarian stages. The results were expressed in units/min.
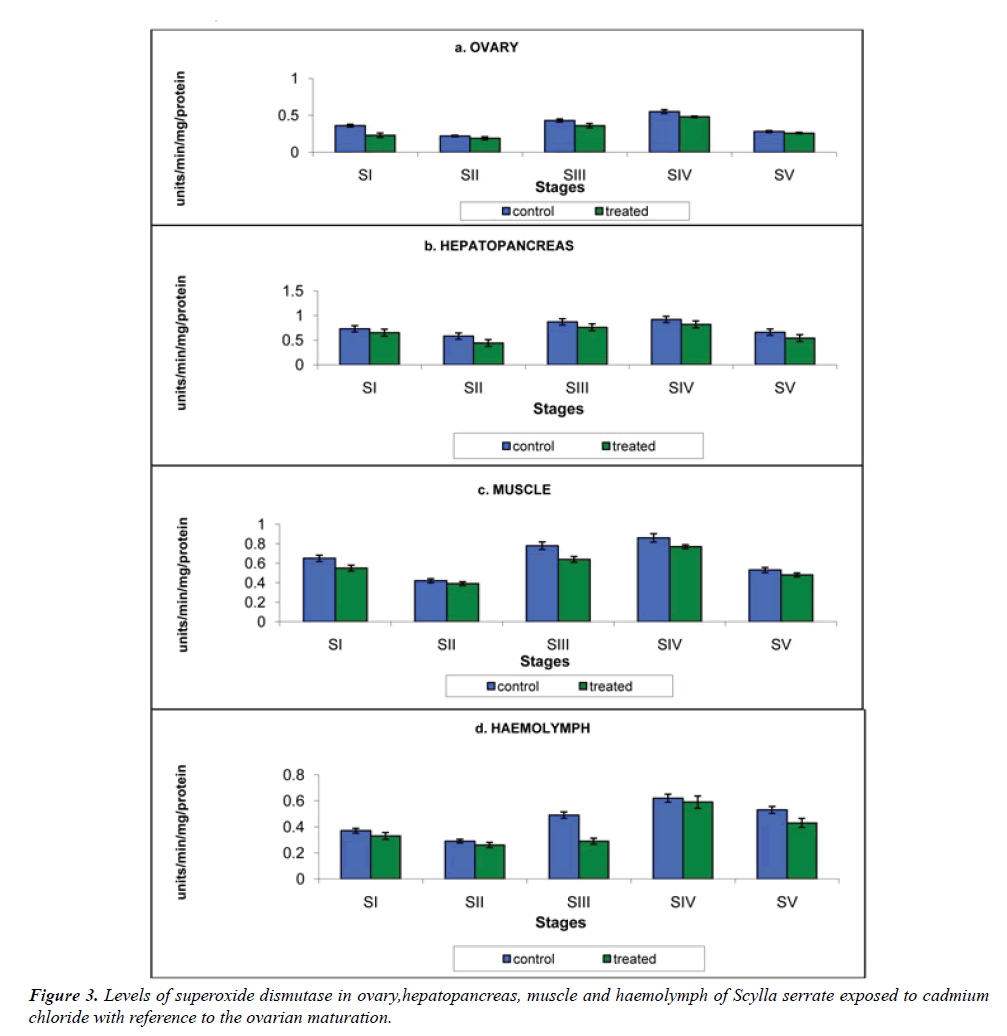
a) Ovary: The activity of ovarian SOD of crabs in the cadmium-free medium was 0.360, 0.220, 0.430, 0.550 and 0.280 units/min. On exposure to cadmium medium it was declined to 0.320, 0.190, 0.360, 0.480 and 0.260 units/min at stages I to V respectively (Figure 3a).
b) Hepatopancreas: Hepatopancreatic SOD enzyme activity was the highest than other tissues and haemolymph. It was 0.73, 0.58, 0.87, 0.92 and 0.66 units/min and on exposure to cadmium it was declined to 0.65, 0.44, 0.76, 0.82 and 0.54 units/min at stage I to V respectively (Figure 3b).
c) Muscle: The SOD activity of the muscle of control Scylla serrata was 0.65, 0.42, 0.78, 0.86 and 0.53 units/min (Figure 3c). This was declined significantly to 0.55, 0.39, 0.64, 0.77 and 0.48 units/min at stages I to V, on exposure to cadmium.
d) Haemolymph: Haemolymph SOD activity in crabs reared in the cadmium-free medium was 0.37, 0.29, 0.49, 0.62 and 0.53 units/min. On exposure to cadmium medium, the reduction in the level of enzyme was 0.33, 0.26, 0.29, 0.59 and 0.43 units/min at stage I to V respectively. (Figure 3d)
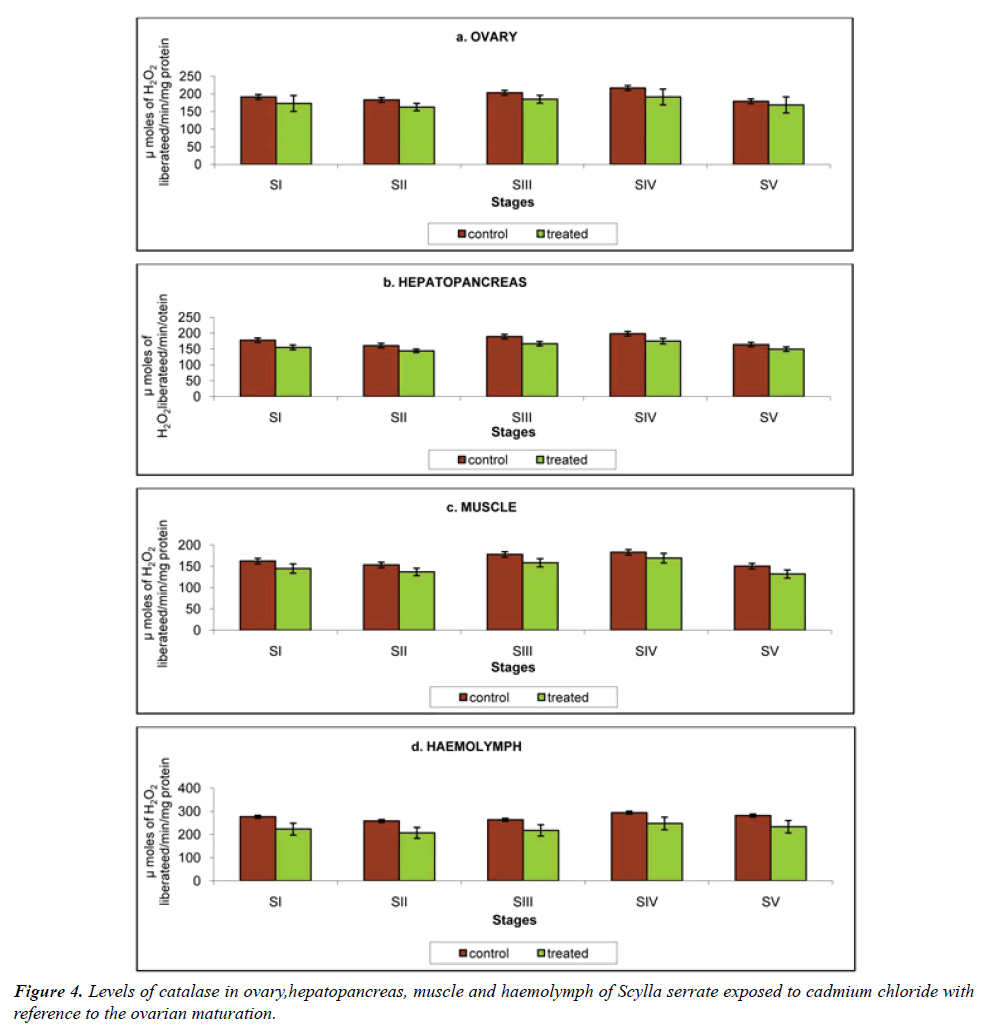
Catalase (CAT): Hematin-containing enzymes catalase facilitate the elimination of hydrogenperoxide (H2O2), which is metabolized to molecular oxygen (O2) and H2O [20] (Table 4).
| Ovarian Developmental stages | Â Ovary | Hepatopancreas | Â Muscle | Haemolymph | |
|---|---|---|---|---|---|
| Stage I | Control | 191.4 ± 10.2 | 177.6 ± 8.3 | 162.3 ± 10.2 | 276.1 ± 11.60 |
| Treated | 173.1 ± 22.6** | 155.3 ± 7.6* | 144.6 ± 10.9** | 223.0 ± 25.78*** | |
| Stage II | Control | 182.9 ± 6.6 | 160.7 ± 6.3 | 153.2 ± 8.5 | 258.0 ± 8.50 |
| Treated | 162.9 ± 10.4* | 144.2 ± 5.4** | 136.8 ± 8.8** | 206.7 ± 23.41*** | |
| Stage III | Control | 203.4 ± 7.9 | 189.1 ± 7.6 | 177.9 ± 9.3 | 263.2 ± 9.64 |
| Treated | 185.0 ± 11.2** | 166.8 ± 6.9** | 158.3 ± 9.7* | 217.6 ± 24.33** | |
| Stage IV | Control | 216.7 ± 11.1 | 198.2 ± 9.2 | 182.8 ± 11.0 | 293.8 ± 13.42 |
| Treated | 191.3 ± 22.6*** | 175.0 ± 8.7*** | 169.1 ± 11.3*** | 247.6 ± 27.12*** | |
| Stage V | Control | 179.1 ± 10.0 | 163.9 ± 8.5 | 150.4 ± 9.0 | 281.3 ± 12.91 |
| Treated | 168.8 ± 22.8** | 149.4 ± 7.3*** | 131.9 ± 9.5*** | 233.2 ± 26.86*** | |
Values were expressed as mean ± SD of 6 observations. Asterisks indicate values that are significantly different from control, *p<0.5, *p<0.01, *p<0.001.
Table 4: Variation in the Catalase activity of ovary, hepatopancreas, muscle and haemolymph in the control and cadmium chloride exposed Scylla serrata in different ovarian stages. The results were expressed in µmoles of H2O2 liberated/min.
a) Ovary: The ovarian catalase activity in crabs reared in the cadmium-free medium was 191.4, 182.9, 203.4, 216.7 and 179.1 μmoles of H2O2 liberated/min. This was declined to 173.1, 162.9, 185.0, 191.3 and 168.8 μmoles of H2O2 liberated/min at stages I to V respectively, when exposed to cadmium (Figure 4a).
b) Hepatopancreas: Hepatopancreatic catalase activity was moderate compared to other two tissues. The hepatopancreatic catalase in control Scylla serrata was 177.6, 160.7, 189.1, 198.2 and 163.9 μmole of H2O2 liberated/min was declined to 155.3, 144.2, 166.8, 175.0 and 149.4 μmoles of H2O2 liberated/min at stages I to V, on exposure to cadmium (Figure 4b)
c) Muscle: In the present investigation catalase activity of the control crabs was recorded to be 162.3, 153.2, 177.9, 182.8 and 150.4 μmoles of H2O2 liberated/min. on exposure to 20 mg/L cadmium, it was reduced to 144.6, 136.8, 158.3, 169.1 and 131.9 μmoles of H2O2 liberated/min at stages I to V respectively (Figure 4c).
d) Haemolymph: Catalase activity recorded in the haemolymph of the control Scylla serrata was the highest; 276.1, 258.0, 263.2, 293.8 and 281.3 μmoles of H2O2 liberated/min. The cadmium concentration used in the present investigation reduced the catalase activity to 223.0, 206.7, 217.6, 247.6 and 233.2 μmoles of H2O2 liberated/min at stages I to V respectively (Figure 4d).
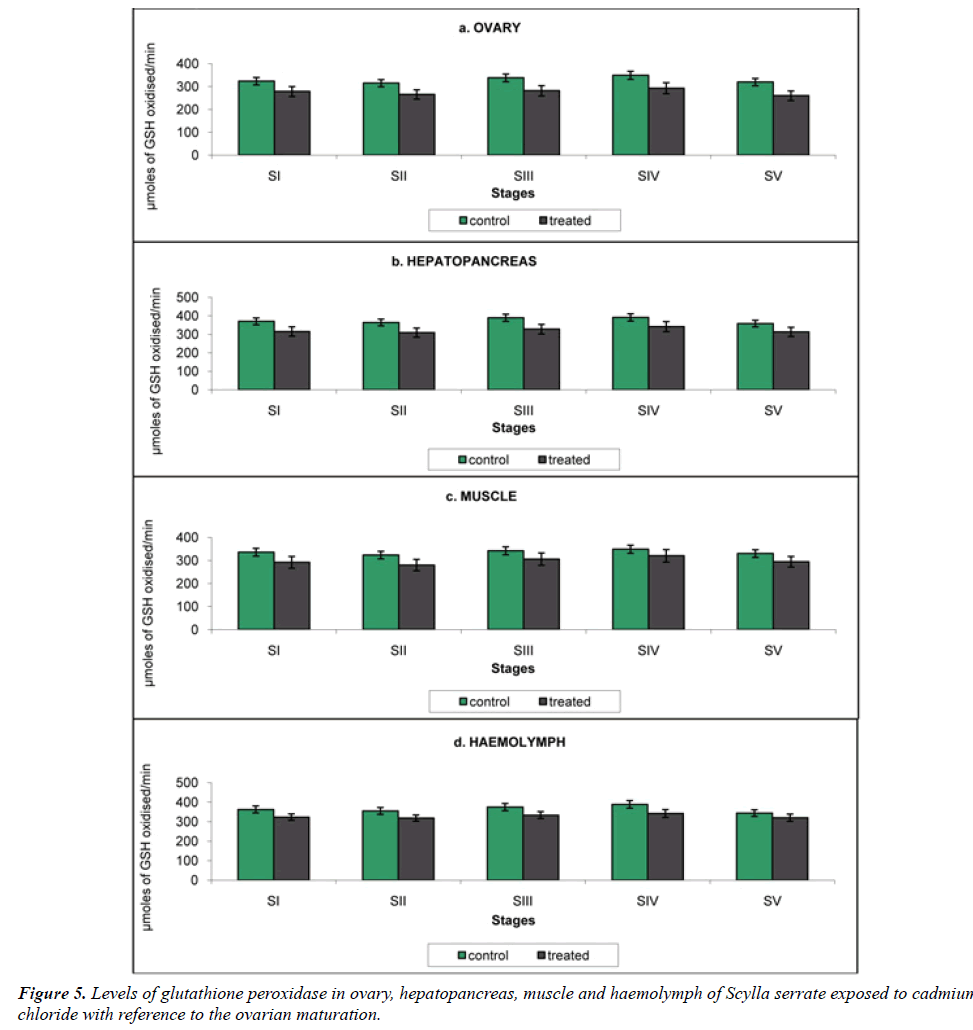
Glutathione peroxidase (GPx): GPx is playinga remarkable character in protecting lipid peroxidation induced membrane damage.GPx catalyzes the metabolism of H2O2 to water, involving a concomitant oxidation of reduced GSH to its oxidized from (GSSG) [21] (Table 5).
| Ovarian Developmental stages | Â Ovary | Hepatopancreas | Â Muscle | Haemolymph | |
|---|---|---|---|---|---|
| Stage I | Control | 323.9 ± 12.89 | 370.3 ± 12.5 | 336.0 ± 19.9 | 362.6 ± 17.8 |
| Treated | 278.6 ± 21.66* | 315.4 ± 15.6*** | 292.0 ± 25.6* | 323.1 ± 16.4** | |
| Stage II | Control | 315.1 ± 11.25 | 364.2 ± 11.9 | 323.5 ± 18.6 | 355.2 ± 16.4 |
| Treated | 265.7 ± 20.58** | 309.4 ± 14.6*** | 280.0 ± 24.7** | 318.2 ± 15.8** | |
| Stage III | Control | 338.2 ± 13.89 | 389.5 ± 13.4 | 342.1 ± 20.4 | 374.6 ± 18.8 |
| Treated | 281.8 ± 22.66** | 328.2 ± 16.7*** | 305.8 ± 26.8** | 333.6 ± 17.9** | |
| Stage IV | Control | 349.6 ± 14.42 | 391.8 ± 14.7 | 348.7 ± 21.7 | 388.7 ± 21.1 |
| Treated | 293.2 ± 23.97*** | 341.6 ± 17.1*** | 320.2 ± 27.1*** | 341.8 ± 20.3*** | |
| Stage V | Control | 320.0 ± 12.05 | 358.2 ± 10.8 | 330.3 ± 17.2 | 343.8 ± 19.3 |
| Treated | 260.0 ± 20.99*** | 312.9 ± 13.3*** | 294.5 ± 23.2*** | 320.0 ± 18.6*** | |
Values were expressed as mean ± SD of 6 observations. Asterisks indicate values that are significantly different from control, *p<0.5, *p<0.01, *p<0.001.
Table 5: Variation in the Glutathione peroxidase activity of ovary, hepatopancreas, muscle and haemolymph in the control and cadmium chloride exposed Scylla serrata in different ovarian stages. The results were expressed in µmoles of GSH oxidised/min.
a) Ovary: Glutathione Peroxidase activity of the ovary was analyzed as 323.9, 315.1, 338.2, 349.6 and 320.0 μmoles of GSH oxidised/min in the control crabs. In crabs reared in cadmium medium, cadmium exposure brought a significant decline in the enzyme activity, 278.6, 265.7, 281.8, 293.2 and 260.0 μmoles of GSH oxidised/min at stages I to V respectively (Figure 5a).
b) Hepatopancreas: The hepatopancreatic GPx activity in the crabs reared in cadmium-free seawater was 370.3, 364.2, 389.5, 391.8 and 358.2 μmoles of GSH oxidised/min declined to 323.1, 318.2, 333.6, 341.8 1nd 320.0 μmoles of GSH oxidised/min at stages I to V respectively on cadmium exposure (Figure 5b).
c) Muscle: GPx activity in the muscle of Scylla serrata in cadmium-free medium was 336.0, 323.5, 342.1, 348.7 and 330.3 μmoles of GSH oxidised/min (Figure 5c). This was declined to 292.0, 280.0, 305.8, 320.2 and 294.5 μmoles of GSH oxidised/min at stages I to V; in the Scylla serrata exposed to cadmium medium.
d) Haemolymph: The haemolymph GPx activity in control crabs was 362.6, 355.2, 374.6, 388.7 and 348.8 μmoles of GSH oxidised/min. On exposure to cadmium it was reduced to 323.1, 318.2, 333.6, 341.8 and 320.0 μmoles of GSH oxidized/min at stages I to v respectively (Figure 5d).
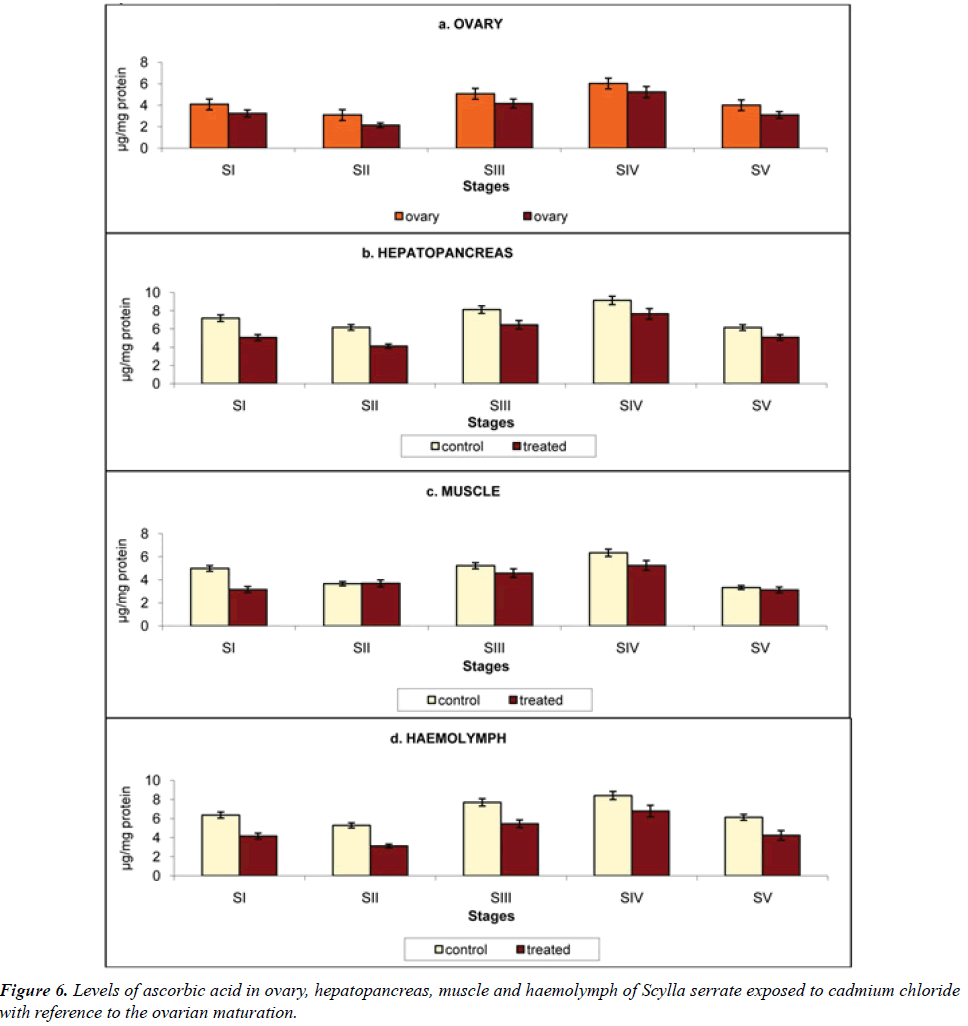
Ascorbic acid: Water-soluble antioxidant ascorbic acid (Vitamin C) save as a cofactor for enzymes involved in collagen biosynthesis or conversions of neurotransmitter [22] (Table 6).
| Ovarian Developmental stages | Â Ovary | Hepatopancreas | Â Muscle | Haemolymph | |
|---|---|---|---|---|---|
| Stage I | Control | 4.08 ± 0.05 | 7.19 ± 0.15 | 4.98 ± 0.04 | 6.36 ± 0.2 |
| Treated | 3.24 ± 0.06* | 5.06 ± 0.33** | 3.16 ± 0.06* | 4.12 ± 0.3** | |
| Stage II | Control | 3.09 ± 0.05 | 6.18 ± 0.14 | 3.66 ± 0.03 | 5.28 ± 0.1 |
| Treated | 2.14 ± 0.06* | 4.12 ± 0.23* | 3.69 ± 0.05* | 3.12 ± 0.2* | |
| Stage III | Control | 5.07 ± 0.04 | 8.13 ± 0.16 | 5.22 ± 0.05 | 7.69 ± 0.3 |
| Treated | 4.16 ± 0.05* | 6.46 ± 0.47** | 4.58 ± 0.06** | 5.45 ± 0.4** | |
| Stage IV | Control | 6.02 ± 0.06 | 9.14 ± 0.17 | 6.34 ± 0.06 | 8.41 ± 0.5 |
| Treated | 5.23 ± 0.07** | 7.66 ± 0.58** | 5.25 ± 0.07*** | 6.78 ± 0.6*** | |
| Stage V | Control | 4.00 ± 0.04 | 6.16 ± 0.14 | 3.33 ± 0.02 | 6.12 ± 0.4 |
| Treated | 3.10 ± 0.05** | 5.08 ± 0.30** | 3.12 ± 0.06** | 4.23 ± 0.5*** | |
Values were expressed as mean ± SD of 6 observations. Asterisks indicate values that are significantly different from control, *p<0.5, *p<0.01, *p<0.001.
Table 6: Variation in the Ascorbic acid activity of ovary, hepatopancreas, muscle and haemolymph in the control and cadmium chloride exposed Scylla serrata in different ovarian stages. The results were expressed in µg/mg protein.
a) Ovary: Influence of cadmium stress on ascorbic acid content in ovary is presented in Figure 6a, it was observed that when animals reared in cadmium-free medium the ascorbic acid content was 4.08, 3.09, 5.07, 6.02 and 4.00 μg/mg protein. On the contrary when the crabs exposed to cadmium the ascorbic acid content was reduced to 3.24, 2.14, 4.16, 5.23 and 3.10 μg/mg protein at stages I to V respectively.
b) Haemolymph: Hepatopancreas registered the highest ascorbic acid content among the tissues analyzed in the present investigation (7.19, 6.18, 8.13, 9.14 and 6.14 μg/ mg protein). This was declined to 5.06, 4.12, 6.46, 7.66 and 5.08 μg/mg protein at stages I to V on exposure to cadmium (Figure 6b).
c) Muscle: In the present investigation, Scylla serrata muscle has 4.98, 3.66, 5.22, 6.34 and 3.33 μg/mg protein of ascorbic acid. It declined to 3.16, 3.69, 4.58, 5.25 and 3.12 μg/mg protein at stages I to V on exposure to cadmium (Figure 6c).
d) Haemolymph: Haemolymph ascorbic acid in the control crabs was 6.36, 5.28, 7.69, 8.41 and 6.12 μg/mg protein which declined to 3.16, 3.69, 4.58, 5.25 and 3.12 μg/mg protein when exposed to cadmium, at stage I to V respectively (Figure 6d).
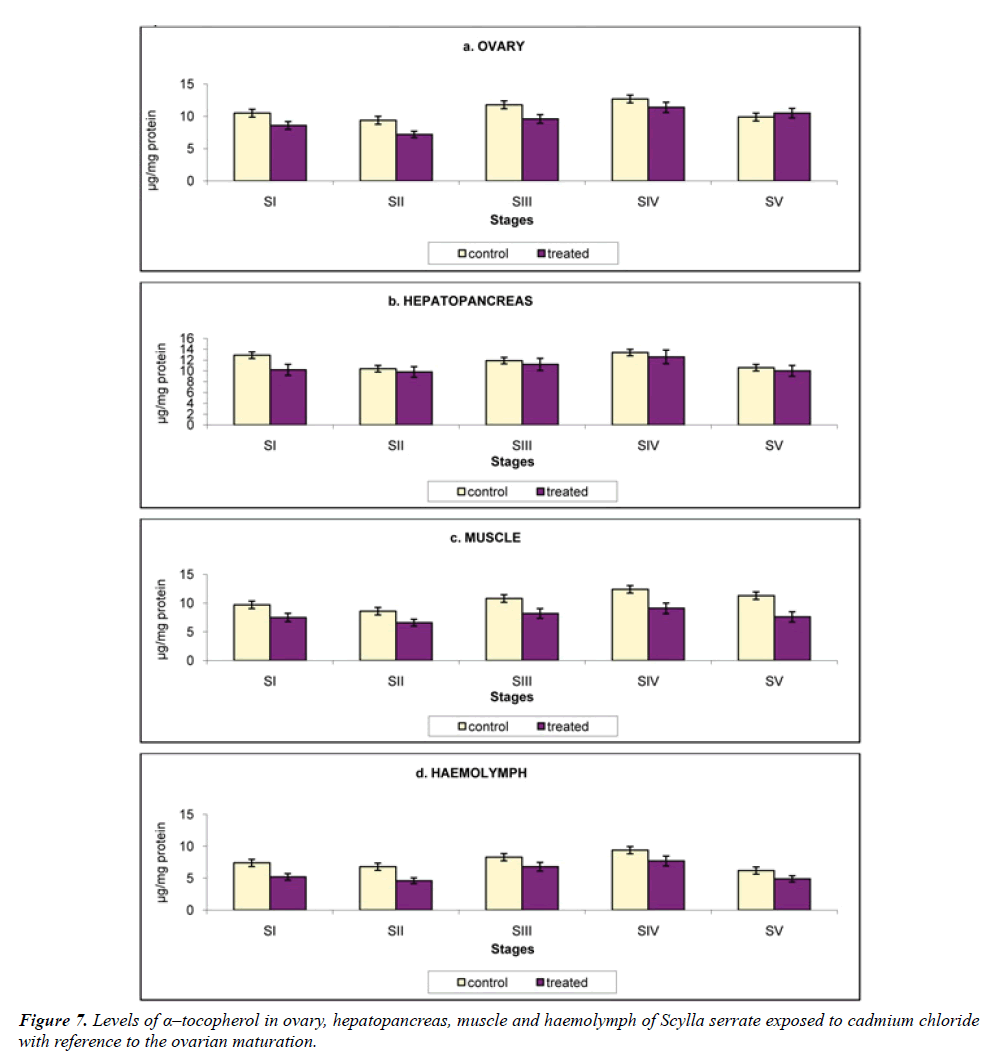
α-Tocopherol: α-Tocopherol or vitamin E, which are multiple, based on their structure. It is also reported to be present in the gonads of both sexes. This is supposed to protect the polyunsaturated fatty acids from oxidation. They stabilize the cellular membrane and play an important role in electron transport. Tissue enzymes are also supposed to be protected by vitamin E [23].
a) Ovary: As the ovary is the tissue were large quantity of lipid is detected in the oocyte, they contain a high level of α-Tocopherol. The control crabs have α-Tocopherol as 4.05, 3.09, 5.07, 6.02 and 4.0 μg/mg protein, which was reduced to 3.24, 2.14, 4.16, 5.23 and 3.1 μg/mg protein, when to exposed to cadmium, at stages I to V (Figure 7a).
b) Hepatopancreas: Hepatopancreas being a lipid depot in crustaceans, like the insect fat body and vertebrate liver it contains the highest α-Tocopherol level 7.19, 6.18, 8.13, 9.14 and 6.16 μg/mg protein at stages I to V respectively. The α-tocopherol level was decreased as 5.06, 4.12, 6.46, 7.66 and 5.08 μg/mg protein when exposed to cadmium (Figure 7b).
c) Muscle: The muscle α-Tocopherol level in control crabs was 4.98, 3.66, 5.22, 6.34 and 3.33 μg/mg protein which declined to 3.16, 3.69, 4.58, 5.25 and 3.12 μg/mg protein at stages I, II, III, IV and V respectively, on exposure to cadmium (Figure 7c).
d) Haemolymph: The level of α-Tocopherolaccounted in the haemolymph was 6.36, 5.28, 7.69, 8.41 and 6.12 μg/mg protein in Scylla serrata reared in cadmium-free medium. On exposure to cadmium the α-Tocopherollevel accounted was 4.16, 3.12, 5.45, 6.78 and 4.23 μg/mg protein (Figure 7d).
Studies on biochemical changes in ovary and other organ systems from the early stage of ovarian development is very important since the ovary starts accumulating the yolk materials from the early stage of ovarian development. The concentration of chemical present in the environment is not adequate for the lethal of the organism whereas sub lethal concentrations modify the biochemistry of biological constituents of the organisms [24]. The metabolic modifications, inhibition of imperative enzymes, hindrance of growth and reproduction and lessening in the fecundity and longevity of the organisms is due to chemical stress induced by environmental toxins [25]. Hence in the present study, Scylla serrata was exposed to 20 mg/L cadmium to study the alterations in the, transaminases, enzymic antioxidants and non-enzymic antioxidants regarding ovarian maturation. Transferases and transaminases are assemblage of enzymes which catalyze the interconversion of aminoacids and α-ketoacids by transfer of amino groups. In the amino transference reactions, the α-ketoglutarate/L-glutamate couple play a vivacious role as an amino group acceptor and donor pair [26]. Alanine transferases catalyzes the transmission of the aminogroup from alanine to α-ketoglutarate to form glutamate and pyruvate, while aspartate transferase catalyzes the transfer of aminogroup from aspartate to α-ketoglutarate to form glutamate and oxaloacetate [27]. In the control animals, the aspartate transaminase and alanine transaminase activity levels were found to be rising from stage I to V. the hepatopancreatic transaminase activity was higher than the ovary, muscle and haemolymph.In thisinvestigation, it has been revealed that alanine transaminase and aspartate transaminase action were amplified in ovary, hepatopancreas, muscle and haemolymph, indicating an increased rate of proteolysis in the tissues [28]. Protein level decline and parallelly transaminases augmented events indicates that, enhanced proteolysis in hepatic tissues and muscle.
Single antioxidant defenses and their comebacks to chemical or biological stress is considered as extraordinary marker in the analysis of oxidative stress. Majority of the environmental chemicals and other toxins basically expose their hazards potential and ends in oxidative damage to the tissues [29]. Inactivation of enzymes, enhanced lipid peroxidation and mutilation to macromolecules like DNA and RNA are the major compliments of oxygen free radicals or oxyradicals when they critically respond with them. These oxyradicals are molecular oxygen reduction products, damage the cellular components and finally stop with cell death [30]. Protecting the cell and tissues from reactive oxygen species induced injury (ROS), detoxification of radicals to nonreactive molecules and boosting the defense mechanism are the primary occupations of antioxidant enzymes. Therefore, the existing investigation was undertaken to examine the result of cadmium, with reference to ovarian maturation. Entirely aerobic cell organization has antioxidant defense arrangements and these involves in the action of neutralizing chemical reactive intermediate formed by endogenous pathways or xenobiotic metabolism. Enzymatic and Nonenzymatic enzymes reaction play an additional role in the electrophilic chemicals eradication and organic peroxides reduction reaction [31]. Alterations and modifications of antioxidant defense mechanism is a significant parameter to analyze the toxicity of the pollutant and their biological impact on human systems. Several investigations demonstrated that, estimation of antioxidants enzymes is a successful method in recognizing or noticing impact of pollutants [32]. In the present investigation, the enzymic and non-enzymic antioxidant systems are reduced in the ovary, hepatopancreas, muscle and haemolymph of exposed Scylla serrata. The level was found rising from Stage I to V and declining at stage V.Enzymatic and non-enzymatic antioxidants are essential for all aerobic organisms to prevent or attenuate the deleterious effects of metal toxicity.
The defensive machineries comprise several enzymatic and non-enzymatic antioxidant enzymes such as supseroxide dismutase (SOD) glutathione peroxidase (GPx), catalase and vitamin C and E Enzymic antioxidants in tissue may be counteracting oxidative stress. SOD catalyses the conversion of superoxide radical (O2-) to hydrogen (H2O2) though catalase (CAT) and Glutathione peroxidase (GPx), converts H2O2 to H2O [33]. These antioxidant enzymes can therefore alleviate the toxic effects of ROS. Oxyradicals initially react with the SOD through quickening the superoxide (O2-) oxyradicals dismutation process to H2O2, which trigger the cellular membrane damage and biological structural modifications. It is well known that, declined levels of SOD is mainly because of increased intracellular ROS activities. Metallo protein SOD is a ubiquities chain breaking antioxidants and is found in all aerobic organisms. Superoxide is converted into H2O2 and the hydrogen peroxide is metabolized and degraded by another antioxidant such as catalase and GPx. Catalase action was repressed by free radicals such as singlet oxygen and peroxyl radicals and it is contained in peroxisomes [34]. In this content, there are opinions that superoxide radicals might prevent the movement of glutathione peroxidase. ROS enhancement might be the motive for declined GPx activity. In the biological system vitamin C and Vitamin E are outstanding non-enzymatic antioxidants and are recognized as second line of defense mechanism against cellular oxidative stress. Over production of ROS was quelled through water soluble antioxidant Vitamin C and protect the DNA and RNA molecules from aqueous radicals generated cell membrane impairment. Noteworthy decline in the activities of nonenzymic antioxidant vitamin C was observed in the present experiment might be due to glutathione enzyme deficiency which performance dehydrosacorbate to ascorbate reduction reaction [35]. Vitamin E also named as α-tocopherol is a biological significant free radical’s scavenger in the cell membrane. Primary function of α-tocopherol is to provide protection from free radical’s damage such as superoxides and H2O2 [36]. Diminished vitamin E activity was detected in the present analysis might be due to increased levels of free radical formation is in accordance with the finding of [37]. From the foregoing account, it can be concluded that protein, carbohydrate, lipid, phophatases, transaminases, enzymic and non-enzymic antioxidants are highly affected when Scylla serrata was reared in the cadmium. Hence, it can be concluded that cadmium affects the vitellogenesis of Scylla serrata.
Conclusion
Cadmium effect in reproductive physiology of estuarine edible crab Scylla serata in relation to ovarian maturation has been analyzed in this experiment. The estimation of tansaminaes and antioxidants were performed in hepatopancreas, muscle and haemolymph in the corresponding stages of ovarian maturation of Scylla serrata after exposure to Cadmium. Cd exhibits biochemical and physiological toxicity for crabs, affecting on activity of antioxidant enzymes alanine Transaminase andaspartate Transaminase. Cd presented noticeable effects on the ovarian maturation of edible crab.
Disclosure of interest
The authors declare that they have no conflicts of interest concerningthis article.
Acknowledgment
Dr. R. Revathy would like to thank Professor and Head, Department of Pharmacology and Environmental Toxicology, University of Madras for providing laboratory and infrastructure facilities to carry out this research work. Dr. V. K. Langeswaran Alagappa University for financial support of RUSA – phase 2.0 grant sanctioned vide Letter No.F.24-51/2014-U policy (TNMulti – Gen), Dept of Edn. Govt of India Dt, 09.10.2018.
References
- Ivanov V, Stabnikov V, Stabnikova O, Kawasaki S. Environmental safety and biosafety in construction biotechnology. World J Microbiol Biotechnol 2019; 35: 26.
- Guo F, Zhang J, Wang C. Enantioselectivity in Environmental Safety and Metabolism of Typical Chiral Organic Pollutants. Curr Protein Pept Sci 2017; 18: 4-9.
- Palanivelu V, Vijayavel K, Balasubramanian SE, Balasubramanian MP. Influence of pesticide-fertilizer combination on food intake, growth, and conversion efficiencies of Oreochromis mossambicus. Bull Environ Contam oxicol 2002; 69: 908-913.
- Borgmann U, Nowierski M, Grapentine LC, Dixon DG. Assessing the cause of impacts on benthic organisms near Rouyn-Noranda, Quebec. Environ Pollut 2004; 129: 39-48.
- Pavlaki MD, Morgado RG, van Gestel CAM, Calado R, Soares AMVM, Loureiro S. Influence of environmental conditions on the toxicokinetics of cadmium in the marine copepod Acartia tonsa. Ecotoxicol Environ Saf 2017; 145: 142-149.
- Reddy PS, Bhagyalakshmi A. Changes in oxidative metabolism in selected tissues of the crab (Scylla serrata) in response to cadmium toxicity. Ecotoxicol Environ Saf 1994; 29: 255-264.
- Vijayavel K, Balasubramanian MP. Changes in oxygen consumption and respiratory enzymes as stress indicators in an estuarine edible crab Scylla serrata exposed to naphthalene. Chemosphere 2006; 63: 1523-1531.
- Vijayavel K, Balasubramanian MP. Reproductive dysfunction induced by naphthalene in an estuarine crab Scylla serrata with reference to vitellogenesis. Ecotoxicol Environ Saf 2008; 69: 89-94.
- Kawada T. Cadmium exposure in inhabitants living in non-polluted area. J Expo Sci Environ Epidemiol 2015; 25: 119.
- Suwazono Y, Kido T, Nakagawa H, Nishijo M, Honda R, Kobayashi E, Dochi M, Nogawa K. Biological half-life of cadmium in the urine of inhabitants after cessation of cadmium exposure. Biomarkers 2009; 14: 77-81.
- Vijayavel K, Anbuselvam C, Balasubramanian MP, Samuel VD, Gopalakrishnan S. Assessment of biochemical components and enzyme activities in the estuarine crab Scylla tranquebarica from naphthalene contaminated habitants. Ecotoxicology 2006; 15: 469-476.
- Arunachalam S, Palanichamy S, Balasubramaniam MP. Effect of carbaryl on esterases in the air breathing fish Channa punctatus (Bloch). Proc Indian Acad Sci (Anim Sci) 1995; 94: 43-77.
- Wooten IDP. Microanalysis of Medical Biochemistry. 4th ed London: J & A Churchill 1964; pp 112-114.
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 1974; 47: 469-474.
- Sinha AK. Colorimetric assay of catalase. Anal Biochem 1972; 47: 389-394.
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hekstra WG. Selenium biochemical role as a component of glutathione peroxidase purification and assay. Science 1973; 179: 588-590.
- Omaye ST, Turnbull JD, Sauberlich HE. Selected methods for the determination of ascorbic acid in animal cells, tissues and fluids. Methods Enzymol 1979; 62: 3-11.
- Desai ID. Vitamin E analysis methods for animal tissues. Methods Enzymol 1984; 105: 138-147.
- Miao L, St Clair DK. Regulation of superoxide dismutase genes: implications in disease. Free Radic Biol Med 2009; 47(4): 344-56.
- Tehrani HS, Moosavi-Movahedi AA. Catalase and its mysteries. Prog Biophys Mol Biol 2018; 140: 5-12.
- Brigelius-Flohe R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta 2013; 1830: 3289-3303.
- Arrigoni O, De Tullio MC. Ascorbic acid:much more than just an antioxidant. Biochim Biophys Acta 2002; 1569: 1-9.
- Traber MG. Vitamin E inadequacy in humans:causes and consequences. Adv Nutr 2014; 5: 503-514.
- Vijayavel K, Anbuselvam C, Balasubramanian MP. Naphthalene?induced hematological disturbances and oxidative stress in an estuarine edible crab, Scylla serrata. Environ Toxicol 2005; 20: 464-466.
- Murthy RC, ALI MM. Effects of in-utero exposure to cadmium on the brain biogenic amine levels and tissue metal distribution in rats. Ind Health 1986; 24: 15-21.
- Moss DW, Henderson AR, Kochmar JF. Enzymes:principles of diagnostic enzymology and the aminotransferases. In:Text book of Clinical Chemistry 1986; 663-768.
- Van der Oost R, Beyer J, Vermeulen NP. Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 2003; 13: 57-149.
- Sastry KV, Shukla V. Influence of protective agents in the toxicity of cadmium to a freshwater fish (Channa punctatus). Bull Environ Contam Toxicol 1994; 53: 711-717.
- Whayne FT, Saha PS, Mukherjee D. Antioxidants in the practice of medicine; what should the clinician know?. Cardiovasc Hematol Disord Drug Targets 2016; 16: 13-20.
- Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem 2015; 97: 55-74.
- Cossu C, Doyotte A, Babut M, Exinger A, Vasseur P. Antioxidant biomarkers in freshwater bivalves, Unio tumidus, in response to different contamination profiles of aquatic sediments. Ecotoxicol Environ Saf 2000; 45: 106-121.
- Livingstone DR. Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull 2001; 42: 656-666.
- Nemmiche S. Oxidative signaling response to cadmium exposure. Toxicol Sci 2016; 156: 4-10.
- Kirkman HN, Gaetani GF. Mammalian catalase: a venerable enzyme with new mysteries. Trends Biochem Sci 2007; 32: 44-50.
- Zwoli?ska D, Grzeszczak W, Szczepa?ska M, Kili?-Pstrusi?ska K, Szprynger K. Vitamins A, E and C as non-enzymatic antioxidants and their relation to lipid peroxidation in children with chronic renal failure. Nephron Clin Pract 2006; 103: c12-8.
- Mustacich DJ, Bruno RS, Traber MG. Vitamin E Vitam Horm 2007; 76: 1-21.
- Bainy AC, Saito E, Carvalho PS, Junqueira VB. Oxidative stress in gill, erythrocytes, liver and kidney of Nile tilapia (Oreochromis niloticus) from a polluted site. Aquat Toxicol 1996; 34: 151-162.