Research Article - Current Pediatric Research (2017) Volume 21, Issue 1
Tissue perfusion assessment of paediatric burns by laser doppler Imaging (LDI).
Mermod T1, Kolly S1, Raffoul W2, El Ezzi O1, Anthony S de Buys Roessingh11Department of Paediatric Surgery, Lausanne University Hospital (CHUV), Switzerland.
2Department of Plastic and Hand Surgery, Lausanne University Hospital (CHUV), Switzerland.
- *Corresponding Author:
- Anthony S de Buys Roessingh
Service de chirurgie pédiatrique, CURCP
Centre Hospitalier Universitaire Vaudois (CHUV)
CH-1011 Lausanne, Switzerland.
Tel: 41 21 314 31 26
Fax: 41 21 314 30 76
E-mail: anthony.debuys-roessingh@chuv.ch
Accepted date: December 27, 2016
Abstract
Introduction: The indication for performing autologous skin grafts in cases of burns in children is based on the clinical examination by trained surgeons and on the progression of spontaneous healing during the first seven to ten days. Spontaneous regeneration of the skin after a burn depends on the presence of tissue microcirculation. Laser Doppler Imaging (LDI) is a non-invasive method for assessing tissue perfusion. The objective of this pilot study was first, to measure tissue perfusion by LDI over the course of the first few days after a burn and second, to show the usefulness of LDI when having to decide whether or not to perform a skin graft. Materials and methods: A prospective pilot study involving children with second-degree burns covering a minimum of 10% of the Total Body Surface Area (TBSA) was conducted over 14 months from May 2012 to July 2013. The treatment of the lesions followed the typical pattern of showers every other day and the use of hydrofibre dressings. LDI images of the burned skin were made under standardized conditions during hydrotherapy over the first ten days and also, at the same time, of healthy skin in order to obtain a reference value. A clinical assessment determining the degree of severity of the burns of each patient was carried out by the paediatric surgeon. The analysis of the results was performed with the EasyLDI Studio program. Results: This pilot study included seven patients. Our results show a correlation between the numerical perfusion values obtained by LDI and the depth of the burn as determined by the surgeon. The perfusion threshold requiring treatment by skin grafting is set at 74% of the perfusion of healthy skin. In 70% of the cases, the sensitivity of the Laser Doppler imager helps determine during the first few days after the burn whether a skin graft will be necessary. Discussion and conclusion: Laser Doppler Imaging (LDI) helps to numerically objectify the residual perfusion of burned tissue. In 70% of the cases, it makes it possible to estimate at an early stage whether a skin graft will be necessary.
Keywords
Imaging, Tissue, Children.
Introduction
Burns are common accidents among the paediatric population and mainly concern children under five years of age or adolescents. They represent the second-highest most common cause of accidental mortality in children. They can be of a diverse nature: thermal, chemical, electrical or by inhalation that accompanies 20-35% of cutaneous burns. The majority of paediatric burns happen at home and in the presence of an adult. The kitchen remains the most common place where accidents occur due to cooking equipment [1]. Lack of regulation is a predisposing factor for accidents referred to as “inevitable”. Hence, the introduction of educational measures (adequate supervision of children, flammable substances kept out of the reach of children and better knowledge of these products), the design of safe appliances and temperature regulation of hot-water outlets have brought a clear reduction in the incidence of accidents [1].
The treatment of burns requires a multidisciplinary course of action over a prolonged period of time. Regular monitoring over months, or even years, by a paediatric surgeon, nursing staff, physiotherapists and ergotherapists is essential. Burns not only entail considerable long-term physical sequelae caused by substantial hypertrophic and retractile scarring, they may also give rise to psychological sequelae due to the circumstances of the accident, the feelings of guilt and the duration of the treatment and aftercare.
Burns are classified according to their depth with respect to the histological layers affected. Burns are therefore considered to be first-degree if they are limited to the epidermis, second-degree, divided into superficial and deep, if limited to the dermis and third-degree when the epidermis and dermis are destroyed down to the subcutaneous fat. Fourth-degree burns or carbonisation, show a complete destruction of the skin, occasionally affecting the underlying structures (ligaments, muscles, bones). It is important to note that a burn is rarely homogeneous and various burn depths may coexist. The extent of the burn also plays an important role in the healing process. Burns covering more than 10% of the Total Body Surface Area (TBSA) of a paediatric patient are considered severe. Finally, burns to face, genitals, hands, feet and joints must be referred to a centre specializing in multidisciplinary care.
Second-degree and more severe deep burns require autologous skin grafts to avoid the development of hypertrophic scars with disabling retractions. For seconddegree burns, which are the most common, the need for a skin graft is generally determined between day seven and day ten post-trauma. Spontaneous tissue regeneration is difficult to predict during the first few days and will depend on the tissue microcirculation [2-9].
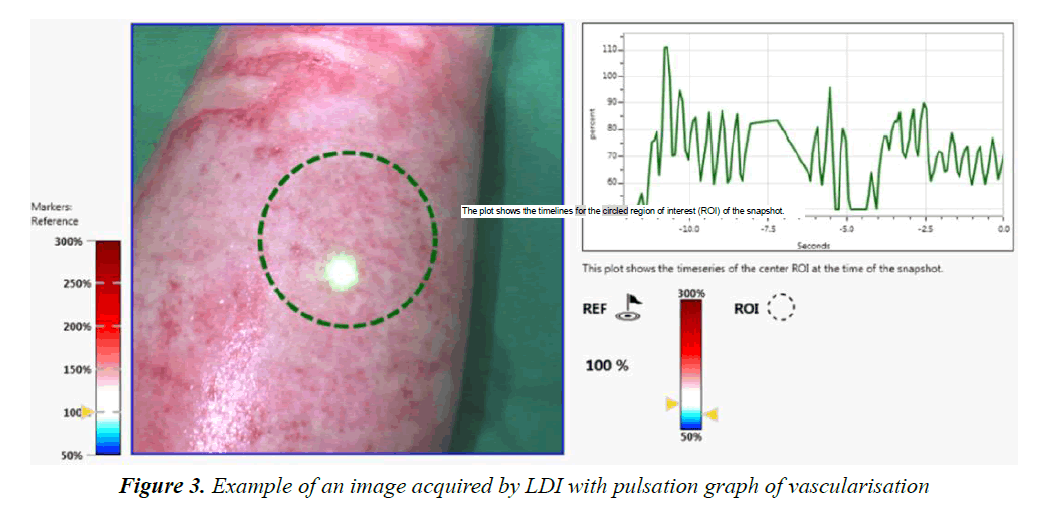
Laser Doppler Imaging (LDI) is a non-invasive macroscopic imaging modality (Figure 1). It requires no contact whatsoever and, through a Doppler system, enables the evaluation of tissue microcirculation [10-17]. Several studies in adults have validated the capability of LDI to predict the progression of burns if it is performed within 48 to 72 h post-incident [10-12,14,17]. It appears particularly useful in supporting clinical judgement on second-degree intermediate burns by providing objective information on tissue perfusion, and reflects the skin’s capacity to spontaneously regenerate itself. In the absence of such perfusion during the first few days of care, excision of the burn together with an early-stage graft would help adjust or even improve, our current clinical protocol by hastening surgical treatment.
In this study, we endeavour to objectify this tissue regenerating capacity and to numerically and objectively measure tissue vascularisation in order to corroborate these findings with our subjective impressions of tissue recovery. The aim is to identify an objective measure of prediction of tissue recovery.
Materials and Methods
This prospective pilot study was conducted from May 2012 to July 2013 at the Department of Paediatric Surgery of the Lausanne University Hospital (CHUV), Switzerland. The study included patients presenting with a burned skin surface area greater than 10% of the TBSA. The study was the subject of an application submitted to the Ethics Committee and required parental authorisation together with a signed Informed Consent Agreement.
Following an initial evaluation including airway examination and an estimate of the percentage of TBSA burned and the depth of the burn, treatment followed the usual pattern, with a tetanus booster shot and bacteriological examination by skin smear: day 1, treatment under sedation or anaesthesia with shower and irrigation using 0.9% sodium chloride and camomile extract at 2% dilution, Flamazine®/Ialugen® cream if the phlyctena are intact or Aquacel Ag® dressing for ruptured phlyctena. Day 3, identical treatment under sedation or anaesthesia with shower and Aquacel Ag® dressing, and first LDI measurement. The images of the burned area were taken with the laser Doppler imager after the shower treatment (after drying the skin of the child), and reference images of healthy skin, ideally contralateral, were also taken. Days 5, 7 and 10, same treatment and same LDI measurement. The decision whether to perform a skin graft was taken on day ten post-trauma. If by day ten the skin had epithelialised and the Aquacel Ag® dressing was adherent, it was not replaced. A graft was not deemed necessary.
The LDI machine (Aïmago® EasyLDI) used in this study
is a third-generation laser Doppler imager developed by
the Laboratory of Biomedical Imaging (LIB) of the Swiss
Federal Institute of Technology in Lausanne (EPFL).
It was approved in Switzerland for the intended use in
this study (microcirculation assessment) [11,17]. It is
equipped with a camera scanning surfaces of 7 × 7 cm
(912 × 900 pixels) at a frame rate of 4 Hz, while emitting
a wavelength of 808 nm adjusted to the wave absorption
by oxyhaemoglobin (HbO2) and deoxyhaemoglobin
(HHb) (Figure 1). The laser Doppler imager is capable of
acquiring up to 40 frames per second, thus overcoming the
shortcomings of older LDI machines, namely insufficient
acquisition speed resulting in distorted perfusion images,
given that the blood flow velocity and hence the perfusion rate vary according to the cardiac cycle. The laser emits a Near-Infrared (NIR) beam with a wavelength
of approx. 800 nm (150 mW) that illuminates the tissue
and is scattered differently according to the two fields,
one static, corresponding to rigid tissues and the other
dynamic, representing tissues in motion, specifically
the erythrocytes. The reflection of the two fields causes
a fluctuation due to the superposition of two frequencies
that are close but not equal. The reflected beams are
detected by the receiver located next to the laser emitter.
The intensity as a function of time I (t) is processed by
Fourier transformation F(I(t)) to yield the values in the
form of spectral lines. The spectral power is determined by S(ν) = (F(I(t))² and from this, the notion of moments that
correlates with the physiological behaviour can be derived.
Thus, the erythrocyte concentration is proportional to the
zero-order moment 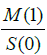 and the perfusion is proportional
to the first-order moment
and the perfusion is proportional
to the first-order moment 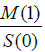 By normalising all values relative to S (0), the effect of
the tissues, i.e., the static component is reduced. The noise
generated from the fluctuation in luminous intensity in
the static field and due the scanning speed is then reduced
by the processing of the signal. The scan of the observed
area produces a real-time colour image/map of the tissue
perfusion.
By normalising all values relative to S (0), the effect of
the tissues, i.e., the static component is reduced. The noise
generated from the fluctuation in luminous intensity in
the static field and due the scanning speed is then reduced
by the processing of the signal. The scan of the observed
area produces a real-time colour image/map of the tissue
perfusion.
The acquisition of the frames is done in the same way as for a digital photograph. The distance between the subject and the device is defined by a light beam and it is important to position the device perpendicularly to the area photographed. Three images of the burned skin were acquired to obtain the average of the measurements. We averaged the measurements provided by two images of healthy skin taken at the same time [18-20].
The acquired frames were analysed with the "EasyLDI Studio" (v1.0-v1.2) program that allowed us to define a quantitative measure in [apu] for the vascularisation of lesions. The value given in [apu] is a unit of measurement that enables us to obtain vascularisation values specific to the patient by comparing the contralateral healthy skin (reference standard) with that of the burn. The method of analysis was the same used in the study: Assessment of the role of LASER-Doppler in the treatment of port-wine stains in infants, Mermod et al. [21].
Statistical Analysis
On each of the four LDI imaging sessions, vascularisation before and after treatment was compared using the Wilcoxon signed-rank test, as the vascularisation did not follow a normal distribution. The same test was used to compare vascularisation between each treatment session.
Results
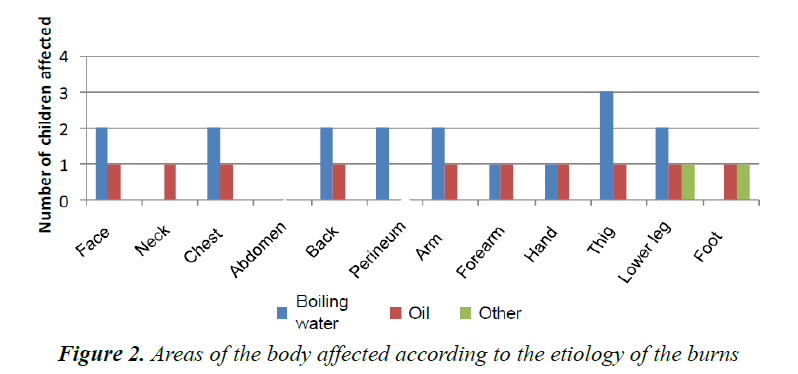
Our results are based on a group of seven patients, four girls and three boys with ages ranging from one month to five years at the time of the accident. Four patients suffered burns from boiling water and two patients from hot oil. One patient had burns from steam. The affected areas were the thighs (four children), the legs (four children), the face (one child), the chest and the arms (three children) (Figure 2); in each child the burn may had happened at several body parts.
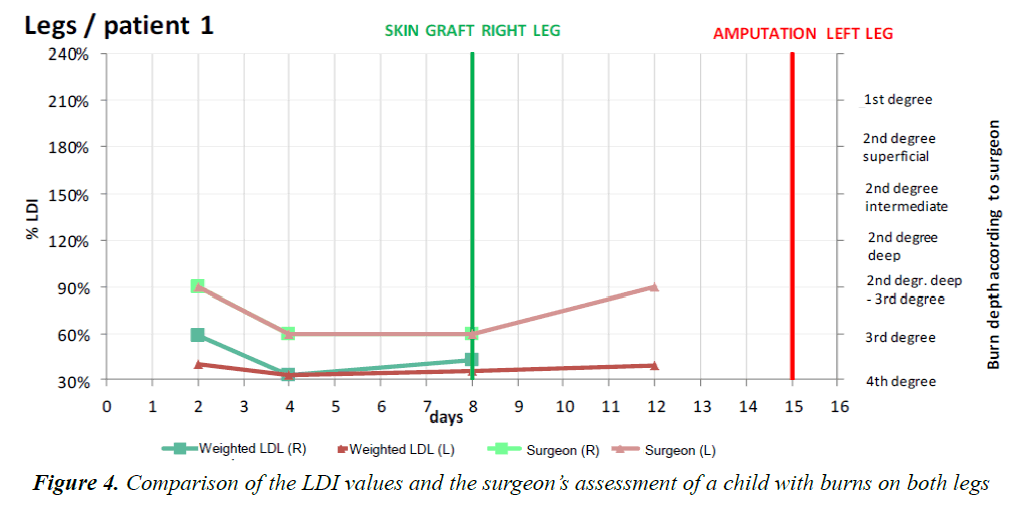
The frames acquired with the LDI machine (Figure 3) were compared to the surgeon’s assessment of each patient and for each part of the body affected. The areas of the body were subdivided as follows: face, neck, chest, abdomen, back, perineum, arm, forearm, hand, thigh, lower leg, foot. Figure 4 shows the progression over time of the microvascularisation determined by LDI as well as the surgeon’s assessment. The values obtained by LDI are comprised between 33% and 59% of vascularisation (healthy skin equivalent to 100%) over the first ten days of treatment. The surgeon’s opinion shifted from an intermediate second-degree burn classification to profound second degree/third-degree as a result of the clinical progression in two children who then received skin grafts.
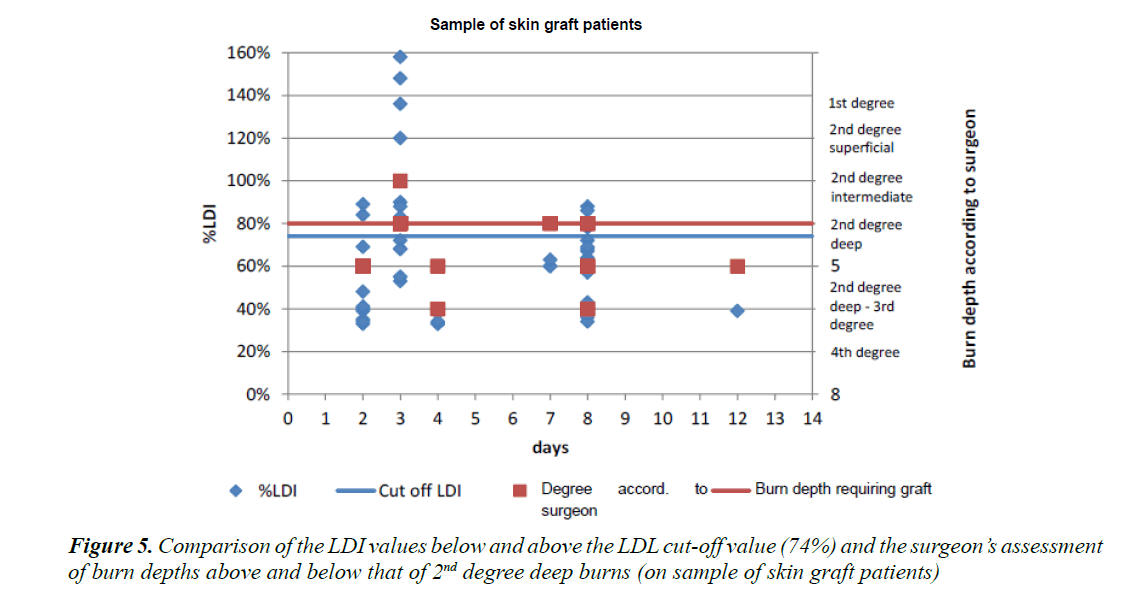
Figure 5 shows the cutaneous microperfusion values in a burned area so that a threshold value for which a skin graft is required can be determined. The experienced surgeon’s opinion on whether or not a skin graft was needed was declared the “gold standard” to be used as a reference standard. We projected the values obtained by LDI in the form of a point cloud as a function of the opinion given by the surgeon at the same time. This enabled us to observe that the numerical vascularisation values tend to corroborate the results of the clinical examination.
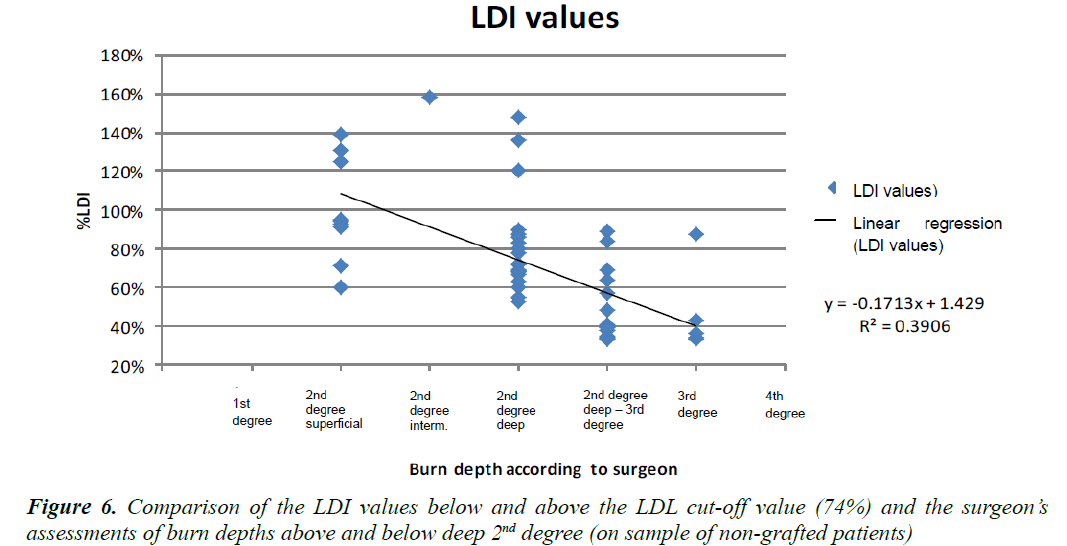
Figure 6 shows a diagram featuring all the patients as a function of the percentage of vascularisation at the level of the burn versus the burn depth as determined by the surgeon. By observing the children who required a skin graft versus those treated in a conservative manner, we were able to establish a cut-off value of 74% of vascularisation for the requirement of a skin graft. All children displaying vascularisation levels superior to the cut-off value could be treated conservatively.
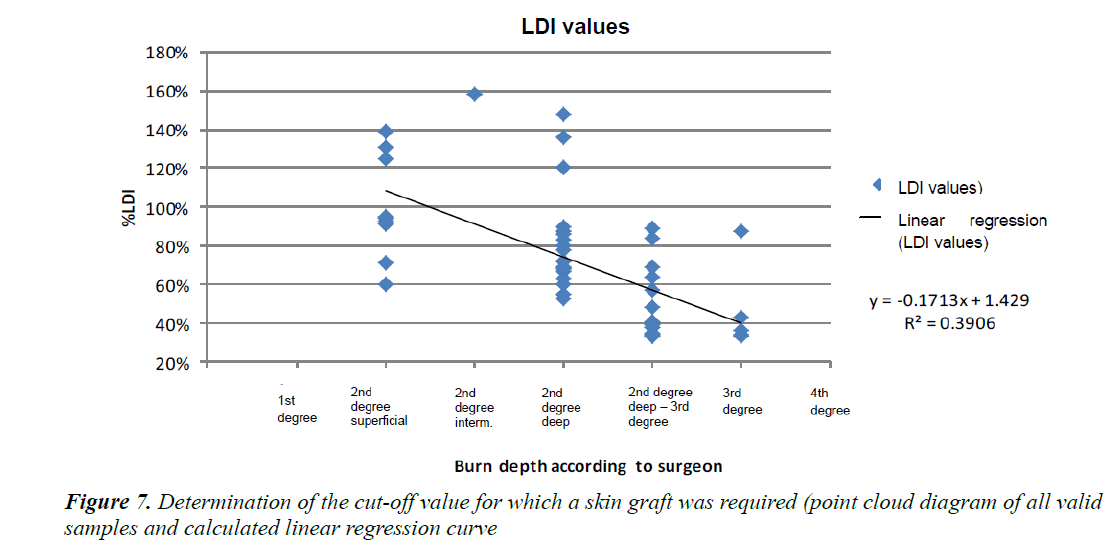
The regression curve satisfying the equation y = −0.1713x +1.429 , the intersection between the above-mentioned curve and the vertical plane passing through the second-degree deep burn corresponding to the “gold standard” threshold for the requirement of a graft gave us the LDI threshold value of 74%. We carried out the same measurements on the patients who did not require a skin graft (Figure 7).
Discussion and Conclusion
Our study enabled us to establish a cut-off value below which the children required a skin graft. It also validated the use of the LDI. LDI revealed a higher percentage of vascularisation in superficial burns than in higher-degree, deeper burns, where it was shown to be markedly lower. LDI also allowed us to predict the treatment outcome of conservative treatment versus skin grafting in 70% of the cases.
Several studies on adults validate the capability of LDI to predict the progression of a burn if it is performed within the first 48 to72 h [10,12,14]. During the first 12 to 24 h, burned skin perfusion is minimal and then at 48 h, the beginning of the repair process is observed. The usefulness of LDI is also emphasised by its capability to rate the progression of second-degree intermediate burns in support of the clinician’s assessment [8]. This grey zone could be clarified by LDI, since in second-degree superficial burns the microcirculation is best preserved, whereas in second-degree deep burns, the main vascular structures are often destroyed, leading to a critical state of peripheral structures easily damaged by the oedema.
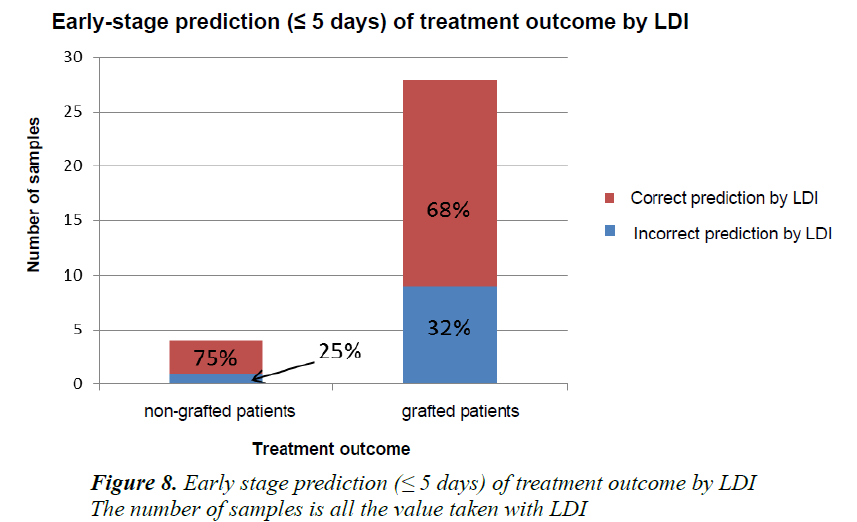
In our study, the treatment outcome was assessed by comparing the microvascularisation values obtained during the first five days post-burn with those evidenced after the final treatment with or without a skin graft. It was observed that in patients who benefited from a skin graft, when relying on the early-stage microvascularisation values of the first five days post-burn, 68% of the patients presented values below the “gold standard” of 74% (cutoff value for the necessity of a graft) required surgical treatment. 75% of the patients who displayed early-stage microvascularisation values above that of the “gold standard” did not in fact require a skin graft. The remaining 25% did however eventually require it (Figure 8).
Nevertheless, we found a relatively low linear correlation coefficient. It describes how the points scatter from the median-median line of best fit. This scattering of points can be explained by various phenomena: the heterogenicity of the burned area photographed the heterogenicity of the healing in the burned area and the operator-dependent differences in quality of the snapshots.
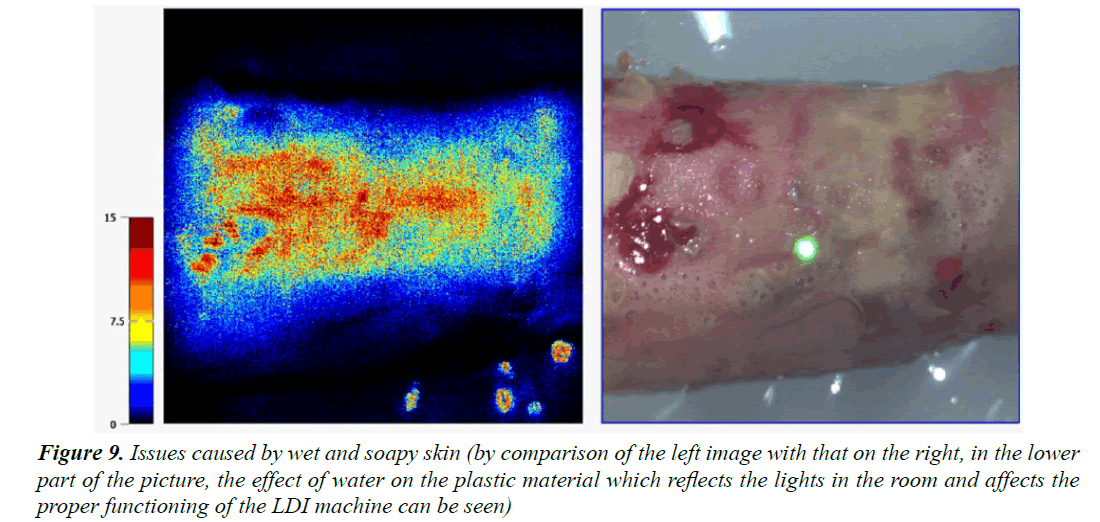
To improve the results, we must first of all take action in regard to the operator-dependent differences in the snapshot quality, i.e., by instructing several people in the use of the LDI. It is important that the snapshots are always taken of the adequate areas in the correct way: at an appropriate distance and at an adequate angle of incidence. The image quality is essential for reducing the acquisition biases. It was noted that emergency situations such as the treatment of burns only leave little time for starting the machine and putting it into operation. As a result, several images were taken in a rush and had to be discarded on account of their being blurred: the distances were inadequate and the light dots used for image acquisition in optimum conditions did not converge exactly, thus reducing the resolution of the images. In addition, certain snapshots were saturated and gave poor pulsation graphs. It is also paramount that the child’s skin be well dried without the presence of soap or water (Figure 9). Operation of the laser Doppler imager by non-experienced persons appears to lead to the loss of relevant and critical information, affecting the quality of the measurements.
These various issues suggest that the measuring protocol needs to be refined to the greatest extent possible, and adhered to as strictly as possible. We therefore propose a short training course for a number of operators and a rigorously systematic approach to be able to clearly compare the data between one occasion and the next. Thus for each patient, right from the first frame, it is necessary to (1) prepare a clear diagram of the burned areas of the body together with the reference areas of healthy tissue, so that each new series of measurements will be repeated on the same areas; (2) clearly map out the areas photographed for the study and repeat the measurements of the same area from one occasion to the next in order to achieve the best comparison possible of a given area over the various days; (3) focus on the total immobilisation of the area photographed to avoid any slow movement which might be imperceptible to the naked eye but which would produce a pulsation graph with a slight drift; (4) numerically record the degree of burn depth after each course of hydrotherapy or change of dressing for which an LDI image was taken to facilitate the comparison of the values relayed by the surgeon to the operator; (5) analyse the data immediately after acquisition to be able to anticipate any corrections that need to be made for the next measurements; (6) take a minimum of three snapshots per burned area to obtain a curve representative of the progression of the case.
It should also be noted that for the areas of the body with a pronounced curvature several snapshots of the same area have to be taken while slightly varying the angle of inclination in order to achieve an average value for the burned area that is as accurate as possible. Only a single image of flatter areas is required with the software program allowing you to move across the entire area photographed and to obtain the various values of perfusion. This is an important point that needs to be made, given the existing stress levels involved in the treatment of burn victims, where time is at a premium and a minimum number of high-quality images will help save time in the operating theatre and the data can rapidly be analyzed afterwards. It is important to emphasize that, for the time being; only a small group of patients has been followed by us.
In conclusion, the LDI is a useful tool that can provide effective assistance in the treatment and continued care of burn patients. It could, in fact, become a valuable aid in determining the need for skin grafts at an early stage. Nevertheless, it has proved to be very operator-dependent, and adequate results can only be achieved by qualified people trained on, and familiar with, the machine.
References
- Peden M, Oyegbite K, Ozanne Smith J, et al. Rapport mondial sur la prévention des traumatismes chez l?enfant. OMS, UNICEF 2008; 211.
- Jeschke MG, Herndon DN. Burns in children: Standard and new treatments. Lancet 2013: 61093-61094.
- De Buys Roessingh A, Holfeld J, Scaletta C, et al. Development, characterization and use of a fetal skin cell bank for tissue engineering in wound healing. Cell Transplantation 2006; 15: 823-834.
- Cho JK, Moon DJ, Kim SG, et al. Relationship between healing time and mean perfusion unit of laser Doppler imaging (LDI) in pediatric burns. Burns 2009; 35: 818-823.
- Kaiser M, Yafi A, Cinat M, et al. Non-invasive assessment of burn wound severity using optical technology: A review of current and future modalities. Burns 2011; 37: 377-386.
- Nguyen K, Ward D, Lam L, et al. Laser Doppler imaging prediction of burn wound outcome in children: Is it possible before 48 h? Burns 2010; 36: 793-798.
- Holland AJA, Martin HCO, Cass DT. Laser Doppler imaging prediction of burn wound outcome in children. Burns 2002; 28: 11-17.
- Sharma VB, Boyle OCP, Jeffery SLA. Man or machine? The clinometric properties of laser Doppler imaging in burn depth assessment. J Burn Care Res 2011; 32: 143-149.
- La Hei ER, Holland AJA, Martin HCO. Laser Doppler imaging of pediatric burns: Burn wound outcome can be predicted independent of clinical examination. Burns 2006; 32: 550-553.
- Mileski WJ, Atiles L, Purdue G, et al. Serial measurements increase the accuracy of laser Doppler assessment of burn wounds. J Burn Care Rehabil 2003; 24: 187-191.
- Leutenegger M, Martin-Williams E, Harbi P, et al. Real-time full field laser Doppler imaging. Biomed Opt Express 2011; 6.
- Monstrey SM, Hoeksema H, Baker RD, et al. Reprint of: Burn wound healing time assessed by laser doppler imaging. Part 2: validation of a dedicated colour code for image interpretation. Burns 2012; 38: 195-202.
- Mill J, Cuttle L, Harkin DG, et al. Laser Doppler imaging in pediatric burns population. Burns 2009; 35: 824-831.
- Hoeksema H, Van de Sijpe K, Tondu T, et al. Accuracy of early burn depth assessment by laser Doppler imaging on different days post burn. Burns 2009; 35: 36-45.
- Monstrey S, Hoeksema H, Verbelen J, et al. Assessment of burn depth and burn wound healing potential. Burns 2008; 34: 761-769.
- Serov A, Steinacher B, Lasser T. Full-field LASER-Doppler perfusion imaging and monitoring with an intelligent CMOS camera. Opt Express 2005; 13: 3681-3689.
- Erba P, Espinoza D, Koch N, et al. Flux explorer: A new high-speed laser Doppler imaging system for the assessment of burn injuries. Skin Res Technol 2012;18: 456-461.
- Shin JY, Yi HS. Diagnostic accuracy of laser Doppler imaging in burn depth assessment: Systematic review and meta-analysis. Burns 2016; 42: 1369-1376.
- Hop MJ, Hiddingh J, Stekelenburg C, et al. Cost-effectiveness of laser Doppler imaging in burn care in the Netherlands. BMC Surg 2013; 13: 2.
- Hoeksema H, Baker RD, Holland AJ, et al. A new, fast LDI for assessment of burns: A multi-centre clinical evaluation. Burns 2014; 40: 1274-1282.
- Mermod T, El Ezzi O, Raffoul W, et al. Assessment of the role of laser Doppler in the treatment of port-wine stains in infants. J Pediatr Surg 2015; 59: 1388-1392.