Keywords
|
| Thymus daenensis, Methanolic extract, Time-kill kinetics, Pharmacodynamics. |
Introduction
|
| The use of plants as medicines to treat various human diseases dates back to ancient times. Iran has one of the richest histories of use of medicinal plants as traditional folk medicine. Different geographical areas and diverse meteorological conditions have made it an appropriate country to host over 8000 species of plants. The traditional knowledge of medical use of many of these plants has been gathered in various local communities [1]. |
| Amongst the medicinal plant families, Labiatae is known as one of the largest groups of medicinal plants, consisting of about 200 genera and 3000 species worldwide [2]. The genus Thymus belonging to Labiatae consists of hardy herbs that are able to adapt to extreme climatic conditions. Of the 215 Thymus spp., 14 species grow wild in the flora of Iran that are locally known as AVISHAN (corresponding to its English name: Thyme) [3]. |
| T. daenensis is a well-known medicinal plant indigenous to Iran. Various medicinal properties have been reported for this herb, focusing mostly on its essential oils. Amongst other pharmacological activities, a number of studies have demonstrated antimicrobial activities for essential oils or crude extracts from leaves and flowers of T. daenensis against various pathogenic fungi and bacteria [4]. However, no study has elucidated time-kill kinetics of antibacterial activity of T. daenensis. In addition, much focus has been given to qualitative tests such as disc diffusion, and there is very limited quantified information on dose-response curves and endpoint parameters particularly bactericidal and bacteriostatic activities. The purpose of this study was, thus, to quantify the antimicrobial activities and time-kill kinetics of T. daenensis methanolic extract against several gram-positive and gramnegative pathogenic bacteria, using a colorimetric microdilution method. |
Materials and Methods
|
Production of methanolic extract
|
| Dried leaves of Thymus daenensis Celak (local name: Avishane- Denaei) confirmed by Rechinger (1963-1998) [5], were purchased from an Iranian agricultural products company (Pakan Bazr, Isfahan, Iran). Extraction was performed as described elsewhere [6]. Shortly, each 30 g of the plant material was ground to a fine powder in a blender and macerated with 300 ml of methanol (Analytical grade, Merck, Darmstadt, Germany). Extraction was performed at room temperature at 150 rpm for 48 hours. The residue was reextracted with additional 100 ml of the same solvent. The extracts were filtered (with Whatman No.1 filter paper), combined and concentrated in a rotating vacuum evaporator (RE 300, Heidolph, Germany) at 40°C, and incubated until dryness. The extracts were stored in airtight containers at 4°C until use. |
Bacterial strains
|
| The following freeze-dried bacterial strains were purchased from the Iranian Biological Resource Center (IBRC): Bacillus cereus (IBRC-M 10796), Staphylococcus aureus (IBRC-M 10690), Escherichia coli (IBRC-M 10698), Psudomonas aeroginosa (IBRC-M 10205), and Enterococcus faecalis (IBRC-M 10740). The other bacterial strains were obtained from the Persian Type Culture Collection as freeze-dried ampoules: Salmonella Typhi (PTCC 1609), Bacillus subtilis subsp. Spizizenii (PTCC 1023), Listeria monocytogenes (PTCC 1298), and Klebsiella pneumoniae (PTCC 1290). Mueller Hinton Broth (MHB) (Scharlau Microbiology, Barcelona, Spain) was used to revive the lyophilized bacteria and maintain bacterial broth cultures. Mueller Hinton Agar II (MHA) (Biolife, Milan, Italy) was utilized to prepare solid cultures of the bacterial strains. |
Antibacterial activity assay
|
| The antibacterial activity of methanolic extract was quantified by a colorimetric broth dilution method [7]. Prior to testing the antibacterial activity, the maximum non-toxic dose of DMSO against the tested bacteria was determined to be 10%. The extract was then re-constituted in 100% DMSO at 400 mg/ml followed by making various serial dilutions in the MHB to reach the range of 0.25-40 mg/ml. To each well, 70 μL of the extract and 70 μL of the bacterial inoculum of 1.5 × 108 cfu/ml was added. Four controls comprising medium with 10% DMSO, medium with bacterial inoculum (negative control), medium with the extract (to measure the turbidity of the extracts at each concentration), and medium only were included in each experiment. All experiments were performed in triplicate and the plates were incubated at 37°C for 24 hours, after which 50 μL of 0.25% (w/v) 2,3,5-Triphenyltetrazolium Chloride (TTC) (Merck, Darmstadt, Germany) was added into each well. The plates were incubated for further 30 minutes. The color change (from clear to red) was observed, photographed and then recorded using a microplate reader (Epoch-Biotek, USA) at 625 nm. The dose-response curves for bacterial inhibition were drawn using the following equation: (1-t/c) × 100, where t represents the absorbance of the bacterial strains treated with the plant extract, from which the absorbance of the extract was subtracted, and c represents the absorbance of the negative control. MIC was defined as the lowest concentration of the plant extract inhibiting the visible growth of a microorganism. MIC values were further confirmed through absorbance reading (producing 95% or more reduction in bacterial viability). MIC50 and MIC90 were determined based on the dose-response curves. Minimum bactericidal concentration (MBC) was defined as the minimum concentration of the extract that was able to cause a 99.9% reduction in bacterial viable count on the MHA. |
Time-kill studies
|
| Kinetics of antiviral activity of the methanolic extract was performed based on a modified method of Sim et al., [8]. The basic experimental conditions were the same as those explained for the antibacterial activity assay. Selection of the extract concentrations was guided by the MIC endpoints. Each 70 μL of the plant extract at various concentrations (MIC, ½ MIC, and ¼ MIC) was added to each well, and 70 μl/well of the bacterial inoculum with the density of 1.5 × 108 cfu.ml-1 was also added. The controls were included the same as for the antibacterial activity. All experiments were performed in triplicate and the plates were incubated at 37°C. Photographic observations and absorbance readings were performed at 30- minute intervals for the first two hours, followed by two-hour intervals for the later 10 hours. Quantification of readings and determination of antibacterial activity was performed as explained before. |
Statistical analysis
|
| All the treatments were applied in triplicate, and each experiment was independently repeated at least three times. Graph Pad Prism version 6 was utilized to conduct statistical analyses and ANOVA tests. Means were compared using Tukey method with a significance level (p value) of 0.05. |
Results
|
Antibacterial activity of the T. daenensis extract
|
| The extract inhibited the growth of all the tested bacterial strains in a dose-dependent manner, along with significant differences among the bacterial strains (P<0.05). As presented in Table 1, except for P. aeroginosa, lower MIC50s were observed for the gram-negative bacteria as compared to the gram-positive bacteria (P<0.05). According to the statistical analysis, less concentrations of the extract was required to inhibit 50% of the growth of Bacillus spp. as well as the most of gram-negative bacterial strains, in comparison to E. faecalis, S. aureus and P. aeroginosa (P<0.05). |
| A similar trend was seen with MIC90 so that the lowest MIC90 values were observed for Bacillus spp. as well as the gramnegative bacteria, including E. coli, L. monocytogenes, and S. Typhi. |
| As compared to the dose-response curves, the endpoint determined MIC values were found to be less different across the tested microorganisms (Table 1). All the bacterial strains, regardless of being gram-positive or gram-negative, were completely inhibited by the plant extract with MIC values of 25-35 and mg/ml. However, when it came to MBC, P. aeroginosa and K. pneumoniae were significantly prevented at higher concentrations as compared to the other bacterial strains (P<0.05). The ratio of MBC/MIC was determined as an additional parameter in this research. An MBC/MIC ≤ 4 was considered to indicate bactericidal character of the extract [8]. Accordingly, the extract had bactericidal activity towards all of the tested bacterial strains. |
Kinetics of antibacterial activity of the T. daenensis extract
|
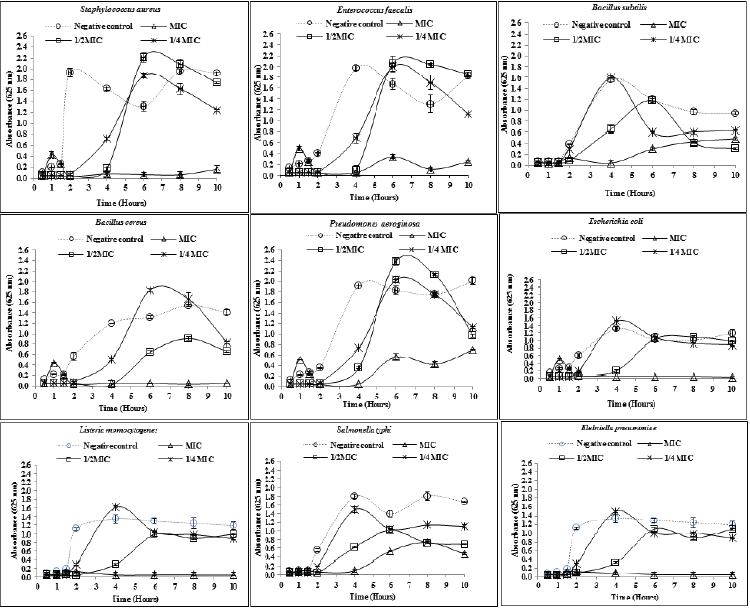
| For each bacterial strain, a separate time-kill profile was produced over a period of 10 hours following inoculation. According to the findings, no measurable bacterial growth was observed for any of the bacterial strains in the first 30 minutes after inoculation. Differences in antibacterial activity among the concentrations or among the bacterial strains were more obvious after one and a half hours post inoculation. At the concentrations corresponding to MIC, E. coli and B. cereus were completely blocked at the shortest time after inoculation (less than two hours), followed by L. momocytogenes and K. pneumoniae which were inhibited at four and six hours post inoculation, respectively. All of these inhibition patterns remained unchanged until the end of the time course. The plant extract showed the bactericidal activity against S. aureus at the time points 2-8 hours, after which the microorganism regrew slightly. However, there was still a very significant difference in bacterial viability between the negative control and the bacterial sample exposed to the plant extract at 10 hours after inoculation (P<0.05). The kill kinetics at MICs for the rest of bacterial strains showed that all of them were almost completely blocked between 2-4 hours post inoculation, after which the bacteria regrew significantly until the end of the time course. |
| At the concentrations corresponding to ½ × MIC, a rapid complete inhibition was observed for E. coli, B. cereus, S. aureus, and E. faecalis. For the rest of the bacterial strains, no complete prevention was observed at ½ MIC at any of the time points. In general, all of the tested bacteria regrew rapidly after four hours exposure to the extract at ½ × MIC. |
| No complete inhibition was observed with the concentrations corresponding to ¼ × MIC at any of the time intervals. However, the extract at ¼ MIC was still able to delay the exponential growing phases of B. cereus, S. aureus, and E. faecalis (Figure 1). |
Discussion
|
| In this study, we quantified the antibacterial effect of the methanolic extract of T. daenensis through an automated, chromogenic endpoint assay, ensuring the turbidity of the plant extract was excluded from that of the bacterial growth. Recently, several studies have reported antibacterial activities of extracts or essential oils of T. daenensis [4]. While these reports have effectively represented the antibacterial activity of T. daenensis, less quantified and clear information regarding dose-response parameters and endpoints such as MBC, and MIC/MBC has been provided. Thus, it is difficult to clearly conclude whether the tested essential oils or extracts of T. daenensis have had bactericidal or bacteriostatic activities. In addition, being solely dependent on qualitative antibacterial activity approaches could possibly be prone to subjective errors. Thus, use of a quantitative test assisted with a chromogenic reagent may improve reliability and reproducibility, as only living microorganisms react with the chromogen reagent [9]. |
| Despite differences in the methods, a simple comparison of the dose-response values (namely MIC50) observed in this study with those of another report showed similarities, where gram negative bacteria were reported to be more sensitive to the ethanolic extract of T. daenensis as compared to gram-positive bacteria [10]. However, our findings might contradict others [11,12], where no antibacterial activity was seen with methanolic extracts of T. daenensis against gram-negative bacteria using disk diffusion methods. |
| Overall, the results obtained with MIC50, MIC90, MIC, MBC, and MBC/MIC suggests that the extract of T. daenensis would be most efficient against the growth of B. cereus and E. coli By contrast, P. aeroginosa could be considered the least susceptible microorganism to the extract. In addition, the extract at the relevant MIC concentrations showed to have bactericidal activity rather than bacteriostatic activity against all of the tested bacteria. These findings may add new information to the previous reported studies, in order to make a better picture of the antibacterial capacity of T. daenensis. |
| For the first time, efforts were made in this study to explore time-kill kinetics of antibacterial activity of T. daenensis. The profiles of killing and re-growth of the tested bacterial strains were assessed over a course of 10 hours post inoculation. Time-kill studies are important because comprehensive information about pharmacodynamics of a putative antibacterial agent may not be gained simply through endpoints such as MIC [13]. Therefore, time-kill assays are required to quantitate pharmacodynamics of a putative antibacterial agent by quantifying the decrease in bacterial growth as a function of time and the drug concentration [8,13]. |
| The time-kill findings in this study displayed levels of time dependent bacterial inhibition that were different among the tested bacteria and the concentrations, regardless of being gram-negative or gram-positive. For example, gram-positive B. cereus and gram-negative E. coli displayed similar time-kill patterns at the concentrations corresponding to MIC and ½ MIC. Also, gram-positive B. subtilis and gram negative S. typhi exhibited similar kinetic patterns at the concentrations corresponding to MIC. These findings might suggest that kinetics of responding of bacterial strains to the T. daenensis extract during the first 10 hours of incubation does not necessarily depend to being gram-negative or gram-positive. |
| According to the results obtained from the endpoint assays and kinetic analysis, B. cereus and E. coli might be suggested the most susceptible bacteria that were inhibited very shortly after exposure to T. daenensis extract. On the contrary, P. aeroginosa could possibly be considered the most resistant microorganism that was not completely inhibited at the relevant MIC before 10 hours after inoculation. |
Conclusion
|
| The findings of this research provided quantitative information on dose-response rate, endpoints, and time-dependent concentrations required to make a significant decrease in the initial bacterial inoculum. These findings may be considered initial steps of in vitro pharmacodynamics of antibacterial activity of T. daenensis. Further investigations are underway to gain deeper insight into mechanism of action and to provide more evidence on the extent of damage of the antibacterial effect of T. daenensis. |
Tables at a glance
|
 |
| Table 1 |
|
Figures at a glance
|
 |
| Figure 1 |
|
References
|
- Behzad S, Pirani A, Mosaddegh M. Cytotoxic activity of some medicinal plants from Hamedan district of Iran. Iran J Pharm Res 2014; 13: 199-205.
- Sharafzadeh S, Alizadeh O. Some medicinal plants cultivated in Iran. J Appl Pharmaceut Sci 2012; 2: 134-137.
- Stahl-Biskup E, Saez F. Thyme, The genus Thymus. Taylor and Francis 2002; 331.
- Zarshenas M, Krenn L. A critical overview on Thymus daenensisCelak.: phytochemical and pharmacological Investigations. J Integr Med 2015; 13: 91-98.
- Rechinger KH. Flora Iranica. Austria, Graz: AkademischeDruck und Verlagsanstalt 1963-1998; Vol. 1-173.
- Rezaeian S-H, Saadatmand S, NejadSattari T, Mirshamsi A. Antioxidant potency of Iranian newly cultivated wild mushrooms of Agaricus and Pleurotus species. Biomed Res 2015; 26: 534-542.
- Martins S, Amorim ELC, PeixotoSobrinho TJS, Saraiva AM, Pisciottano MNC, Aguilar CN, Teixeira JA, Mussatto SL. Antibacterial activity of crude methanolic extract and fractions obtained from Larreatridentata leaves. Ind Crops Prod 2013; 41: 306-311.
- Sim J-H, Jamaludin NS, Khoo C-H, Cheah Y-K, Abdul Halim SNB, Seng H-L, Tiekink ERT. In vitro antibacterial and time-kill evaluation of phosphanegold(I) dithiocarbamates, R3PAu[S2CN(iPr)CH2CH2OH] for R = Ph, Cy and Et, against a broad range of Gram-positive and Gram-negative bacteria. Gold Bull 2014; 47: 225-236.
- Jorgensen JH, Ferraro MJ. Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin Infect Dis 2009; 49: 1749-1755.
- Ghasemi Pirbalouti A, Malekpoor F, Enteshari S, Yousefi M, Momtaz H, Hamedi B. Antibacterial activity of some folklore medicinal plants used by Bakhtiari tribal in Southwest Iran. Int J Biol 2010; 2: 55-63.
- Mojab F, Poursaeed M, Mehrgan H, Pakdaman S-H. Antibacterial activity of Thymus daenesismethanolic extract. Pak J Pharm Sci 2013; 21: 210-213.
- Khukhan K. Harmful activities of microorganisms and essential oil composition of Thymus daenensisCelak from Iran. Front Agric Food Technol 2013; 1: 075-078.
- Schaper K-J, Schubert S, Dalhoff A. Kinetics and quantification of antibacterial effects of beta-lactams, macrolides, and quinolones against gram-positive and gram-negative RTI pathogens. Infection 2005; 33: 3-14.
|
