Research Article - Biomedical Research (2017) Volume 28, Issue 16
Therapy with belimumab may suppress the response of peripheral blood mononuclear cells to apoptotic cells
Yi Liu1#, Pei Yin2# and Bing Yan1*1Department of Rheumatology, West China Hospital, Sichuan University, Chengdu, PR China
2The First Affiliated Hospital, Zhejiang University, Hangzhou, PR China
#These two authors contributed equally to this study.
- *Corresponding Author:
- Bing Yan
Department of Rheumatology, West China Hospital
Sichuan University, Chengdu, PR China
Accepted date: July 24, 2017
Abstract
Belimumab, on top of background Systemic Lupus Erythematosus (SLE) standard therapy, is effective in reducing SLE disease activity and preventing lupus flares, which was confirmed in “BLISS trials”. The mechanisms involved in preventing lupus flare by belimumab remain largely unknown. We aimed to explore the response of PBMCs from lupus patients under belimumab treatment to the apoptotic cells. PBMCs, obtained from five lupus patients with disease flare treated with belimumab, were coincubated with the apoptotic cells induced from Jurkat cells treatment. After incubation, the cells were harvested for surface markers analysis by flow cytometry and the supernatants were tested for cytokines assayed by ELISA kits. Before belimumab treatment, Peripheral Blood Mononuclear Cells (PBMCs) from lupus patients showed markedly secretion of Interferon-α (IFNα) and Interleukin-6 (IL-6) in response to the apoptotic cells. After belimumab treatment, lupus PBMCs were characterized by significant reduction of IFNα secretion (P=0.0103) upon recognition of the apoptotic cells. Reduced activation was also observed in lupus monocytes and T lymphocyte. Belimumab treatment suppressed response of lupus PBMCs to the apoptotic cells, which provides a fresh clue to understand mechanism other than B-cell depletion in the prevention of lupus flare.
Keywords
Systemic lupus erythematosus (SLE), B-cell-activation factor, Belimumab, Interferon alpha (IFNα), Apoptosis.
Introduction
Systemic Lupus Erythematosus (SLE) has a relapsingremitting course with disease activity flares over time. Flare is common, occurring in 65-70% of patients with SLE within 1 y even when they are receiving standard SLE therapy [1]. The environmental triggers for lupus flare, for example ultraviolet light and infection, were suggested to mediate the apoptotic and necrotic cells production [2]. SLE is characterized by a myriad of immunological abnormalities involving defective clearance of apoptotic materials [3]. The accumulated cellular remnants, including nuclear autoantigens and nucleosomes, might consequently change tolerance and lead to flare.
B-lymphocyte activity plays a pivotal role in the development and course of SLE. B-Cell-Activation Factor (BAFF) is an essential factor for B cell maturation, survival, proliferation and immunoglobulin class switching. Belimumab is a fully humanized monoclonal antibody that specifically binds to the soluble BAFF and prevents BAFF interaction with its receptors.
Belimumab was approved by the US FDA for lupus therapy in 2011. Supported by the randomized controlled trials, the addition of belimumab to standard therapy was confirmed to be effective in reducing SLE disease activity and delaying the time to lupus flares [4]. The longer term efficacy of belimumab as maintenance treatment to prevent lupus flares has to be established with extended observational studies [5,6]. As for the mechanism, previous study showed belimumab therapy reduced the number of circulating naive B cells, activated B cells and plasma cells, but not the number of circulating memory B cells [7]. However, the mechanisms other than Bcell depletion remain an open question.
Given PBMC’s availability from SLE patients and its extensive use in auto-immune disorders, we used PBMCs in the study. Here, we revealed suppressed responses of Peripheral Blood Mononuclear Cells (PBMCs) from lupus patients under belimumab treatment to the apoptotic cells. We also observed: (1) IFNα secretion was significantly decreased; (2) The deactivation of monocytes and T lymphocytes.
Patients and Methods
Patients
Five SLE patients enrolled in the present study visited department of rheumatology every 1 to 6 months in the West China hospital. Ethics approval for this study was granted by the Medical Ethics Review Board of West China Hospital, Sichuan University School. Informed consent was obtained from all study subjects. All studies were performed according to the Declaration of Helsinki.
All patients fulfil ≥ 4 of the 1997 American College of Rheumatology classification criteria for SLE. Flare was defined as an increase in SLE Disease Activity Index 2000 update (SLEDAI-2K) with score of ≥ 4 from the previous visit [8,9]. On top of background SLE therapy, they received belimumab at the dosage of 10 mg/kg to be given intravenously at 2 week intervals for the first three doses, followed by 4 week intervals.
Monoclonal antibodies
For immunostaining and analysis by Fluorescence-Activated Cell Sorting (FACS), we used fluorochrome-conjugated mouse Monoclonal Antibodies (mAb) against human CD14-PE, CD80-PE-Cy7, CD86-APC, CD3-PE, CD69-PE-Cy7, CD25- APC, and appropriate isotype controls (all from BD PharMingen, SanDiego, CA).
Preparation for apoptotic Jurkat cells
For induction of apoptosis, Jurkat cells (5 × 106 cells/mL) were treated with 100 nM staurosporine (Beyotime, CN) for 24 h at 37°C in a humidified 5% CO2 atmosphere. Apoptotic and necrotic cell death were confirmed by staining with propidium iodide/annexin V-FITC (Figure 1).
Assay for PBMCs response to apoptotic Jurkat cells
Human PBMCs were freshly isolated by Ficoll densitygradient centrifugation. Labelled with 2 μM CFSE (Invitrogen), PBMCs (5 × 106 cells) were co-incubated with apoptotic Jurkat cells (5 × 106 cells) in 24-well tissue culture plates for 24 h at 37°C. After incubation, cells were harvested for flow cytometry analysis using FC500 (Beckman Coulter, USA). Data were analyzed using CXP software (Beckman). The supernatants were tested for cytokines. Levels of TNFα, IFNα, IFNβ, IFNγ, IL-6, IL-17 and IL-21 were determined by commercially available ELISA kits (R and D Systems).
Statistical analysis
Comparisons between the two groups (T0 and T12w) were performed by paired student’s t test. Statistical analyses were performed using Prism 5.0 software (GraphPad Software, San Diego, CA). P values less than 0.05 were considered significant.
Results
Baseline characteristics
Baseline demographics and disease characteristics of SLE patients were shown in (Table 1). Treatment with belimumab for 12 weeks reduced the disease activity.
| Patients | Sex | Age (y) | Disease duration (y) | Flare episodes | SLEDAI-2K score | Background medication | |
|---|---|---|---|---|---|---|---|
| T0 | T12W | ||||||
| No. 1 | M | 26 | 3 | Cutaneous vasculitis | 12 | 4 | Prednisone 10 mg qd, HCQ 200 mg bid, Aza 50 mg qd |
| No. 2 | F | 22 | 1.5 | Rash, arthritis, cutaneous vasculitis | 20 | 6 | Prednisone 10 mg qd, HCQ 200 mg bid |
| No. 3 | F | 45 | 3 | Proteinuria, thrombcytopenia | 9 | 4 | Prednisone 15 mg qd, HCQ 200 mg bid, CTX 50 mg qd |
| No. 4 | F | 25 | 0.5 | Rash, arthritis, proteinuria | 10 | 4 | Prednisone 50 mg qd, HCQ 200 mg bid, MMF 500 mg bid |
| No. 5 | F | 19 | 3 | Cutaneous vasculitis | 12 | 4 | Prednisone 7.5 mg qd, HCQ 200 mg bid, Aza 50 mg qd |
Table 1: Baseline demographics and disease characteristics of SLE patients.
Altered cytokines secretion from lupus PBMCs in response to apoptotic cells after belimumab treatment
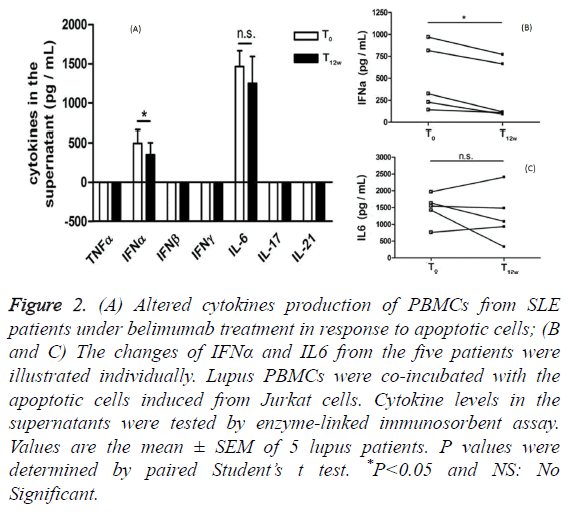
To determine whether PBMCs from lupus patients under belimumab treatment have a distinct pattern of cytokines secretion in response to apoptotic cells co-incubation, we measured the production of several cytokines using an assay, in which lupus PBMCs were co-incubated with the apoptotic cells induced from Jurkat cells. Before belimumab treatment, prominent IFNα and IL-6 secretions (mean ± SD: 494.6 ± 166.5 pg/mL vs. 1465 ± 198.3 pg/mL) were detected in the supernatants when lupus PBMCs were exposed to the apoptotic cells. After belimumab treatment, lupus PBMCs significantly reduced IFNα secretion, but not IL-6 secretion, in response to the apoptotic cells co-incubation (Figure 2A). It is noted that PBMCs from these five lupus patients showed the unanimous declined tendency of IFNα secretion other than IL-6 secretion in the presence of the apoptotic cells (Figures 2B and 2C).
Figure 2: (A) Altered cytokines production of PBMCs from SLE patients under belimumab treatment in response to apoptotic cells; (B and C) The changes of IFNα and IL6 from the five patients were illustrated individually. Lupus PBMCs were co-incubated with the apoptotic cells induced from Jurkat cells. Cytokine levels in the supernatants were tested by enzyme-linked immunosorbent assay. Values are the mean ± SEM of 5 lupus patients. P values were determined by paired Student’s t test. *P<0.05 and NS: No Significant.
Reduced activation of lupus monocytes and T lymphocytes in response to apoptotic cells coincubation after belimumab treatment
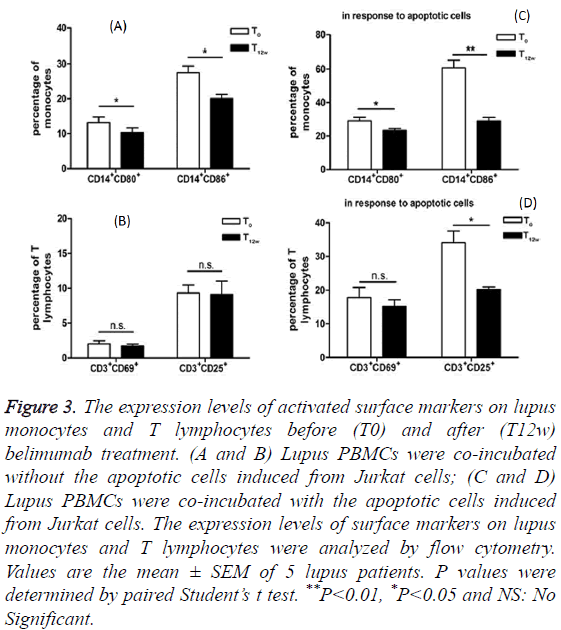
Monocytes from SLE patient’s blood were found to function as antigen-presenting cells. With an increased expression of costimulatory molecules CD80 and CD86, lupus monocytes are efficient stimulators of naive allogeneic CD4+ T cell proliferation [10]. We detected these two surface markers of lupus monocytes and compared their expression levels between before and after belimumab treatments. As shown in Figures 3A and 3C, the expression levels of CD80 and CD86 on the surface of lupus monocytes decreased after belimumab treatment. Furthermore, when exposed to the apoptotic cells, the monocytes from lupus patients under belimumab treatment still showed much lower expression levels of CD80 and CD86 compared to those from the patients prior to the belimumab treatment (Figure 3C).
Figure 3: The expression levels of activated surface markers on lupus monocytes and T lymphocytes before (T0) and after (T12w) belimumab treatment. (A and B) Lupus PBMCs were co-incubated without the apoptotic cells induced from Jurkat cells; (C and D) Lupus PBMCs were co-incubated with the apoptotic cells induced from Jurkat cells. The expression levels of surface markers on lupus monocytes and T lymphocytes were analyzed by flow cytometry. Values are the mean ± SEM of 5 lupus patients. P values were determined by paired Student’s t test. **P<0.01, *P<0.05 and NS: No Significant.
As for the responses of T lymphocytes to the apoptotic cells co-incubation, CD69, the early activation marker of T lymphocytes, did not show the differentiated expression between before and after belimumab therapy (Figure 3B). However, CD25 expression, the late activation marker, remarkably decreased on T lymphocytes from lupus patients after they received belimumab therapy (Figure 3D).
Discussion
These five lupus patients with disease flare presented the improvement in disease activity and declined SLEDAI scores when they received belimumab therapy for 12 weeks. Interestingly, our data revealed that lupus PBMCs showed the suppressed response to the apoptotic cells co-incubation after belimumab treatment, especially IFNα secretion. This may provide a new perspective for understanding the mechanism of preventing lupus flare by belimumab.
Apoptosis plays a key role in pathophysiology of SLE [11]. When autoimmune B cells attack the body’s own tissues, they are normally undergoing apoptosis. In SLE, B cells acquired the ability to survive and proliferate, which protect them from cell death. Particularly, B-cell Activating Factor (BAFF) is required for the development and survival of B cells and BAFF is overexpressed in SLE patients [12]. Type I interferon’s (IFNs) plays an important role in the pathogenesis of SLE. There is cross-talk among type I IFNs, Toll-Like Receptors (TLR) and BAFF [13]. Several studies indicated that the presence of IFNα could influence the expression of BAFF. For instance, induction of BAFF expression was described in myeloid cells, such as Dendritic Cells (DCs) and macrophages, after in vitro stimulation with IFNα [14,15]. A recent study revealed that IFNα-treated SLE monocytes exhibited higher intracellular levels of BAFF, which was mobilized to the membrane rapidly and subsequently released [16]. However, excess BAFF could increase auto-reactive B-cell survival and proliferation, followed by elevating auto-antibodies production and forming immune complexes (ICs). SLE-ICs, especially interferonic ICs, may contribute to unwanted production of IFNα through TLR7/9 in plasmacytoid DCs. Thus, IFNα and BAFF are acting in a vicious circle. Treatment of SLE patients with anti-IFNα mAb could reduce BAFF expression in whole blood [17]. Our study further suggested that treatment of SLE patients with belimumab significantly reduced IFNα secretion in PBMCs upon recognition of apoptotic cells. Given expression of BAFF is controlled by IRF transcription factors and BAFF expression is directly induced by type I IFNs via IRF1 and IRF2, reduced IFN-α secretion may suggest there’s a feedback loop in IRF-IFNs-BAFF signalling [18]. IRF regulates BAFF expression via type 1 IFNs while IFN-α may also be regulated by BAFF when belimumab is present. In addition, levels of CD80 and CD86 dramatically decreased in lupus monocytes in response to co-incubation with apoptotic cells, after treatment with belimumab. The activation status of T lymphocytes showed the same tendency as well as monocytes. These two phenomena may be associated with the decreased level of IFNα.
In summary, our study indicated that PBMCs from the five SLE patients under belimumab treatment showed the suppressed response to the apoptotic cells. The samples size in this study is limited, but it provides clue of the possible mechanism of belimumab’s role in preventing lupus flare (other than B-cell depletion): antagonizing effects on the response to co-incubation with apoptotic cells, such as inhibiting IFNα secretion. Thus, further study with larger sample size is required to deepen our understanding of belimumab’s phamalogical actions. It’s also intriguing to explore whether other SLE drugs (such as hydroxychloroquine, azathioprine and cyclophosphamide) have similar actions to regulate response of cytokine secretion under apoptotic stimuli.
Acknowledgment
The work was supported by the grant (81172870) from the National Nature Science Foundation of China (NSFC).
Conflict of Interest
The authors declare they have no conflicts of interest.
References
- Petri M, Singh S, Tesfasyone H, Malik A. Prevalence of flare and influence of demographic and serologic factors on flare risk in systemic lupus erythematosus: a prospective study. J Rheumatol 2009; 36: 2476-2480.
- Mak A, Tay SH. Environmental factors, toxicants and systemic lupus erythematosus. Int J Mol Sci 2014; 15: 16043-16056.
- Shao WH, Cohen PL. Disturbances of apoptotic cell clearance in systemic lupus erythematosus. Arthritis Res Ther 2011; 13: 202.
- Parodis I, Axelsson M, Gunnarsson I. Belimumab for systemic lupus erythematosus: a practice-based view. Lupus 2013; 22: 372-380.
- Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, Li EK, Thomas M, Kim HY, Leon MG. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011; 377: 721-731.
- Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzova D, Sanchez-Guerrero J, Schwarting A, Merrill JT, Chatham WW. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheumatism 2011; 63: 3918-3930.
- Wallace DJ, Stohl W, Furie RA, Lisse JR, McKay JD, Merrill JT, Petri MA, Ginzler EM, Chatham WW, McCune WJ. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Rheumatism 2009; 61: 1168-1178.
- Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002; 29: 288-291.
- Yee CS, Farewell VT, Isenberg DA, Griffiths B, Teh LS, Bruce IN, Ahmad Y, Rahman A, Prabu A, Akil M. The use of systemic lupus erythematosus disease activity index-2000 to define active disease and minimal clinically meaningful change based on data from a large cohort of systemic lupus erythematosus patients. Rheumatol 2011; 50: 982-988.
- Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Sci 2001; 294: 1540-1543.
- Magna M, Pisetsky DS. The role of cell death in the pathogenesis of SLE: is pyroptosis the missing link? Scandinavian J Immunol 2015; 82: 218-224.
- Do RK, Chen-Kiang S. Mechanism of BLyS action in B cell immunity. Cytokine Growth Factor Rev 2002; 13: 19-25.
- Vincent FB, Morand EF, Schneider P, Mackay F. The BAFF/APRIL system in SLE pathogenesis. Nature Rev Rheumatol 2014; 10: 365-373.
- Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nature Immunol 2002; 3: 822-829.
- Panchanathan R, Choubey D. Murine BAFF expression is up-regulated by estrogen and interferons: implications for sex bias in the development of autoimmunity. Mol Immunol 2013; 53: 15-23.
- Lopez P, Scheel-Toellner D, Rodriguez-Carrio J, Caminal-Montero L, Gordon C, Suarez A. Interferon-alpha-induced B-lymphocyte stimulator expression and mobilization in healthy and systemic lupus erthymatosus monocytes. Rheumatol 2014; 53: 2249-2258.
- Yao Y, Richman L, Higgs BW, Morehouse CA, de los Reyes M, Brohawn P, Zhang J, White B, Coyle AJ, Kiener PA. Neutralization of interferon-alpha/beta-inducible genes and downstream effect in a phase I trial of an anti-interferon-alpha monoclonal antibody in systemic lupus erythematosus. Arthritis Rheumatism 2009; 60: 1785-1796.
- Sjostrand M, Johansson A, Aqrawi L, Olsson T, Wahren-Herlenius M, Espinosa A. The expression of BAFF is controlled by IRF transcription factors. J Immunol 2016; 196: 91-96.