Research Article - Journal of Biochemistry and Biotechnology (2023) Volume 6, Issue 3
Therapeutic potential of Astragalus membranaceus saponins in osteoarthritis through Inhibition of Cartilage Matrix Degradation
Su-Fen Huang1, Ching-Hui Ou2, Han-Wen Yang1, I-Chu Tang3, Wen-Liang Chang3*, and Tsu-Chung Chang1*
1Department of Biochemistry, National Defense Medical Center, Taipei, Taiwan.
2Department of Anesthesiology, Cheng-Hsin General Hospital, Taipei, Taiwan.
3School of Pharmacy, National Defense Medical Center, Taipei, Taiwan.
- Corresponding Author:
- Tsu-Chung Chang
Department of Biochemistry
National Defense Medical Center, Neihu, Taiwan
E-mail: tcchang@mail.ndmctsgh.edu.tw
Received: 25-May-2023, Manuscript No. AABB-23-98103; Editor assigned: 29-May-2023, PreQC No. AABB-23-98103(PQ); Reviewed: 12-Jun-2023, QC No. AABB-23-98103; Revised: 17-Jun-2023, Manuscript No. AABB-23-98103(R); Published: 26-Jun-2023, DOI:10.35841/aabb-6.3.147
Citation: Huang SF, Ou CH, Yang HW, Tang IC, Chang WL, Chang TC. Therapeutic potential of Astragalus Membranaceus saponins in osteoarthritis through Inhibition of Cartilage Matrix Degradation. J Biochem Biotech. 2023;6(3):147
Abstract
Osteoarthritis (OA) is a degenerate joint disease that is characterized by the breakdown of articular cartilage Extracellular Matrix (ECM). Inflammation is a key factor in the development of OA, leading to increased levels of Matrix Metallo Proteinases (MMPs) and destruction of collagen II. A potential therapy for OA would aim to restore ECM production in chondrocytes and prevent cartilage degradation. Astragalus, a traditional Chinese medicine used for centuries to treat a variety of ailments, was investigated in this study for its ability to protect cartilage chondrocytes. Here, the herbal preparation of Astragalus Membranaceus Saponins (AMS) was found to have no apparent toxicity to human chondrocytes and chondrosarcoma cells. AMS significantly increased the uptake of glucosamine, Hyaluronic Acid (HA), and proline in primary human chondrocytes. Using IL-1β-induced in vitro OA model, AMS effectively countered the inflammatory challenge induced by IL-1β in HCH cells by enhancing collagen II synthesis. Furthermore, AMS significantly inhibited the inflammation-induced MMPs expression and proteolytic activities, thereby protecting cartilage ECM from degradation. Overall, the beneficial effects of AMS on MMP, collagen, and HA expression levels in chondrocytes indicate its potential as a therapeutic candidate in preventing cartilage degeneration and joint tissue disorders
Keywords
Astragalus Membranaceus, articulate cartilage, chondrocytes, matrix metalloproteinase, collagen II.
Introduction
Articular Cartilage (AC) is a highly specialized connective tissue of diarthrodial joints. It is a smooth connective tissue that covers the surface of bones where they meet to form joints. Its primary function is to provide a smooth, shock-absorbing and lubricated surface for joint movement. AC is principally composed of a matrix of collagen fibers, proteoglycans, and water, with chondrocytes, the cartilage cells, embedded within the matrix. The matrix provides a framework for the cartilage and the chondrocytes maintain the matrix and produce new Extracellular Matrix (ECM) components [1,2]. Chondrocyte is the only specialized cell type found in AC and which is metabolically active cell that plays a unique role in the development, maintenance, and repair of the ECM [3]. It should be noted that AC has a limited capacity for intrinsic healing and repair. In this regard, it is critical important for maintaining chondrocytes in normal activity in order to keep the healthy function of AC.
In normal AC, the collagen fibers provide tensile strength to
the matrix, while proteoglycans, which are composed of a protein core and long chains of sugars, provide resistance to compression. The proteoglycans in AC are particularly large and complex, giving the tissue its unique properties. Collagen is the most abundant structural macromolecule in ECM, and it makes up about 60% of the dry weight of the cartilage [3,4]. Type II collagen represents 90% to 95% of the collagen in ECM and forms fibrils and fibers that intertwine with the proteoglycan aggregates. Many other collagen types are also present but contribute only to a minor proportion. These minor collagens help to form and stabilize the type II collagen fibril network [4].
Osteoarthritis (OA), affects millions of people worldwide, is the most prevalent chronic and degenerative joint disease. The prominent physiological characteristic of OA is the erosion of AC. In OA, the cartilage becomes thin and brittle, leading to pain, stiffness, accompanied by joint dysfunction [5]. Inflammation plays a major role in OA, particularly through the cytokine Interleukine-1β that stimulates the production of Reactive Oxygen Species (ROS) and Matrix Metallo Proteinases (MMPs) synthesis and secretion in the chondrocytes, leading to breaking down the cartilage ECM, loss of collagen and proteoglycans, and subsequent degradation of cartilage [6]. In addition, IL-1β is also an important mediator of inflammatory pain. Moreover, IL-1β is capable of inducing chondrocyte apoptosis, which further contributes to the breakdown of the joint cartilage tissues. Along with IL-1β, other inflammatory mediators, such as tumor necrosis factor α (TNFα) and interleukin 6 (IL-6) are also involved in stimulating the production of MMPs [7,8]. Although the precise mechanisms of inflammation in OA are still not fully understood, evidence indicates that targeting inflammation represent a promising strategy for managing OA.
Astragalus membranceus (Huangqi) is one of the most popular medicinal herbs for reinforcing vital energy in traditional Chinese medicine [9]. They are included as key components in many traditional therapeutic formulas and as dietary supplements. The main constituents of Astragalus extract include flavonoids, polysaccharides, polyphenols, and cycloartane-type saponins. Astragalus and astragalosides have been used as anti-inflammatory, immune-modulatory, anti-aging, and wound-healing agents [10]. Recently, the clinical and pharmacological effects of astragalus have been extensively investigated. Overall, astragalus is generally considered safe, non-toxic, and effective herbal supplements. Given the marked anti-inflammatory and antioxidant activities of astragalus, the therapeutic potential of the enriched fraction of Astragalus Membranaceus Saponins (AMS) was investigated in chondrocytes. This study will provide valuable information for the development of novel and effective natural therapeutic agent for OA.
Material and Methods
Materials
AMS is the dried extract of Astragalus membranaceus roots blended with maltodextrin as an excipient and its production is compliant with current Good Manufacturing Practice. The standardized AMS sample was provided by NuLiv Science USA Inc (Brea, CA, USA).
Cell culture
The primary Human Chondrocytes (HCH) were purchased from Promo Cell (GmbH, Heidelberg, Germany) and the cells between passages 4 and 10 were used for subsequent experiments. Human chondrosarcoma SW1353 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) containing
4.5 g/L glucose, 0.584 g/L glutamine, 10% fetal bovine serum, 3.7% sodium bicarbonate, 100 IU/ mL penicillin, 100 μg/mL streptomycin, and 1% nonessential amino acids. These cartilage derived cells are widely used model for studying OA or other joint disorders. In this study, AMS was dissolved in Dimethyl Sulfoxide (DMSO) to get stock solutions. The AMS working solutions were prepared by 1:1000 dilution of the stock solutions into culture medium to make final AMS concentrations in 0.1% DMSO. The AMS-containing medium is clear, which were filter sterilized before applying to the cells. For experimental analysis, HCH or SW1353 cells were plated in 6-cm culture dishes at a density of 1 x 106 cells/dish for 12 h prior to treatment with AMS for 24 h before harvested for analysis. Control cells were maintained in medium supplemented with the 0.1% DMSO, which did not show significant cytotoxicity compared to cells not exposed to DMSO. For in vitro OA model, the inflammatory cytokine IL-1β (10 ng/mL) is added to the cell culture medium for 8 h prior to follow-up studies.
Cell viability and proliferation assay
Cell viability assay was carried out using a cell counting kit (CCK-8; Dojindo, Japan). Briefly, 3 × 103 cells/well of HCH or SW1353 cells were seeded onto 96-well plates and allowed to attach overnight. The cells were incubated with or without the indicated concentrations of AMS for 24 h. Then, the original medium was removed, and 10 μl of CCK-8 in 90 μl of phosphate-buffered saline (PBS) was added and incubated for 2 h. The metabolic activation of CCK-8 was quantified by measuring the absorbance at 450 nm using a spectrophotometer. The percentage of cell viability was calculated and compare to control.
Western blot analysis
Western blot analysis was performed using both of the conditioned cultured medium or cell lysates. The cells were plated in 6-cm dishes at a density of 1 x 106 cells/dish for 24 h prior to treatment with indicated concentrations of AMS as indicated for another 24 h. The conditioned culture mediums were then centrifuged and the conditioned medium was 2-fold concentrated and assayed for MMP1, MMP2, MMP9 and MMP13 secreted into the culture medium. For intracellular proteins, the cells were washed and lysed in 0.2 ml of lysis buffer (1% NP-40, 50 mm Tris-Cl, pH 7.4, 180 mm NaCl, 1 mm EDTA, 1 mm PMSF, 1 mm NaF, 10 mm Na3VO4 ) for 30 min at 4℃. After centrifuging at 17,500g for 15 min, the cell lysate supernatants were also saved for analysis. Protein concentration of the samples were measured using the bicinchoninic acid (BCA) protein assay kit according to the manufacturer’s protocol (Pierce, Rockford, IL, USA). Equal amount of protein samples of concentrated conditioned medium or cell lysate supernatants were mixed with an appropriate volume of 4x SDS sampling buffer and separated by 8 % SDS-PAGE gel and blotted to a PVDF membrane. Blotted nitrocellulose was washed and blocked in freshly prepared Tris-buffered saline (TBS) containing 0.1% Tween 20 and 7% skim milk (TBST) for 2 h at room temperature. The nitrocellulose membrane filters were then incubated with either antibodies against collagen II (sc-7764, Santa Cruz Biotechnology, Santa Cruz, CA, USA), MMP1 (GTX-100534, GeneTex, Hsinchu, Taiwan), MMP13 (GTX-59793, GeneTex) or the housekeeping protein α–tubulin (2144, Cell Signaling, Danvers, MA, USA) for 18 h at 4°C. Horseradish peroxidase- conjugated anti-goat antibody (Abcam, Cambridge, UK) was used as secondary antibody. Signals were visualized by an enhanced chemiluminescence kit (Clonetech, Palo Alto, CA, USA) followed by exposure to X-ray films.
Hyaluronic acid (HA) analysis
The cells were grown to high density in 24-well plates. Immediately prior to experiments, cells were washed twice with serum-free medium to completely remove the pre- existing HA accumulated during cell growth. Subsequently, the cells were treated with or without AMS in 0.5 ml serum- free medium for 24 h. The culture medium was collected, centrifuged at 15000g for 5 min, and supernatants were analyzed for HA using an Enzyme-Linked Immunosorbent Assay (ELISA) kit (Echelon Bioscience, Salt Lake, UT).
Glucosamine uptake analysis
In glucosamine uptake test, cells were seeded into 24-well plate at a density of 3 x 104 cells/well and cultured for 24 h. The cells were then treated in the absence (solvent control) or presence of AMS for another 24 h. The treated cells were then washed twice with PBS and incubated in glucose and serum free medium (GSFM). After 2 h, the cells were replaced with fresh GSFM containing 0. 2 μCi of [14C]-Glucosamine (ARC, St. Louis, MO, USA). At designated time interval, the cells were washed twice with GSFM containing cold glucosamine and then lysed in 200 μl 2% SDS. Cell lysates were centrifuged at 15000 g for 15 min. Intracellular uptake glucosamine was determined by transferring 10 μL of the cell lysate to filter- bottomed Unifier plates (Perkim-Elmer, Santa Clara, CA, USA) and counted. Protein concentration of the samples were measured using the BCA protein assay kit. Glucosamine accumulated in the cells was calculated and normalized to protein concentration and uptake rate are expressed as counts per minute per microgram of cellular protein (cpm/min/μg).
Zymographic analysis of MMPs
The proteolytic activities of MMPs from HCH or SW1353 cells were measured essentially as described (11). The cells were seeded for 24 h and then treated with AMS for another 24 h. The conditioned culture medium was then collected, concentrated and assayed for MMPs expressed into the culture medium. The cells were then lysed in lysis buffer (1% Triton, 50 mm Tris-Cl, pH 7.4, 180 mm NaCl, and 1 mm EDTA). Protein concentration of the samples was measured using the bicinchoninic acid (BCA) protein assay kit. Samples of concentrated conditioned medium were mixed with non- reducing electrophoresis loading buffer and subjected to electrophoresis under non-reducing conditions, which was carried out on a 10 % SDS-PAGE co-polymerized with 2 mg/ ml gelatin or casein (Sigma, St. Louis, MO. USA). Following electrophoresis, denaturation of the proteins was achieved by incubating the gels in 25 g/L Triton X-100 at room temperature twice for 10 min. Subsequently the gels were incubated in 50 mm tries-HCl, pH 7.5 containing 0.2 M NaCl, 0.02 % Brij35 and 10 mm CaCl2 at 37 °C for 18 h. After staining the gels with Coomassie Brilliant Blue R-250 and destaining, zones of Proteolytic activities became visible as transparent bands in the stained gel. This procedure allows the visualization of faint gelatinise bands at 92, 125 and > 200 kDa. To measure the activities of the detected MMP enzymes, zymograms were read using a Molecular Dynamics (Sunnyvale, CA, USA) computing laser densitometer with Image Quant software.
Proline uptake assay
The proline uptake assay was carried out as described [12]. Briefly, cells were seeded into 24-well plate at a density of 3 x 104 cells/well and cultured for 24 h. The cells were then treated in the absence (solvent control) or presence of various concentrations of AMS for another 24 h. The treated cells were then washed with PBS and incubated in amino acid free medium (AAFM) for another 30 min. The treated cells were them replaced with fresh AAFM containing 50 μg/ mL of ascorbate and total of 0.5 mM of L-proline containing 1μCi [3H]-Proline (ARC, St. Louis, MO, USA). At designated time interval, the cells were washed with AAFM containing cold proline and then lysed in 200 μL 2% SDS. Cell lysates were centrifuged at 15000 g for 15 min. Intracellular uptake proline was determined by transferring 10 μL of the cell lysate to filter-bottomed Unifier plates (Perkim-Elmer) and counted. Protein concentration of the samples was measured using the BCA protein assay kit. Proline accumulated in the cells was calculated and normalized to protein concentration and uptake rate are expressed as mole of L-proline per minute per milligram of cell protein (mole/min/μg).
Results
AMS displays no apparent cytotoxicity in human chondrocytes
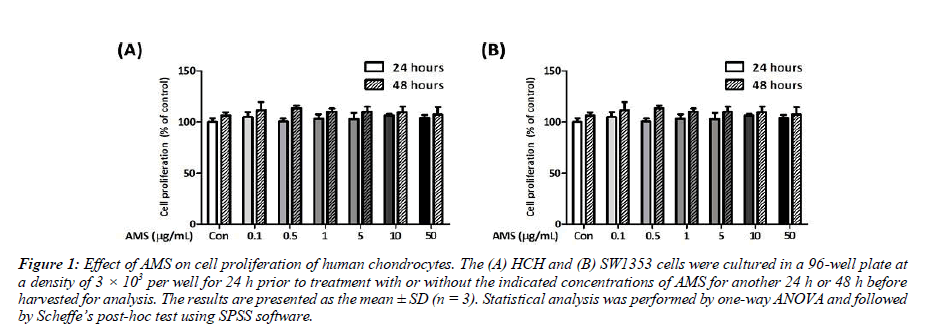
In order to examine the effect of AMS on human chondrocyte viability, HCH or SW1353 cells were treated with increasing concentrations of AMS for 24 or 48 h. Results shown in Figure 1, there is no apparent difference in cell proliferation and viability in all concentrations tested for the AMS-treated HCH or SW1353 cells treated for 24 or 48 h, respectively. As AMS displays no apparent cytotoxicity to both cell models at all concentrations used, the AMS dose at 1 μg/mL was used for further analysis in this study.
Figure 1: Effect of AMS on cell proliferation of human chondrocytes. The (A) HCH and (B) SW1353 cells were cultured in a 96-well plate at a density of 3 × 103 per well for 24 h prior to treatment with or without the indicated concentrations of AMS for another 24 h or 48 h before harvested for analysis. The results are presented as the mean ± SD (n = 3). Statistical analysis was performed by one-way ANOVA and followed by Scheffe’s post-hoc test using SPSS software.
AMS increases glucosamine uptake, HA secretion, and proline uptake in human chondrocytes
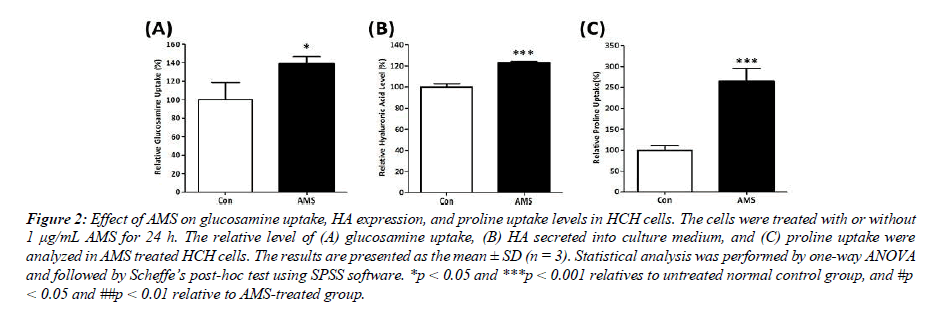
Glucosamine is the primary building block of HA in chondrocytes, which is an essential component in ECM and cartilage tissues formation. In order to verify the chondroprotective activity of AMS, the effect of AMS on glucosamine uptake and HA expression level was examined. Results shown in Figure 2 indicate that the presence of AMS significantly increased glucosamine uptake by 39% (p < 0.05 vs control cells) and enhanced HA level by 23% (p < 0.001 vs control cells) in chondrocytes. These results indicate that AMS significantly facilitate HA synthesis in chondrocytes and providing a protective effect to the cartilage tissue. In addition to HA, collagen is also a chief component in ECM of cartilage tissue. Proline is the primary constituent amino acid that is required for collagen synthesis in cartilage. The measurement of proline uptake is widely used as a marker of cartilage collagen synthesis. As shown in Figure 2, AMS significantly enhanced proline uptake by 265% in human chondrocytes (p< 0.001 vs control cells). These results suggest the potential of AMS in promoting collagen II synthesis, and indicate the potential efficacy of AMS in preventing cartilage degeneration.
Figure 2: Effect of AMS on glucosamine uptake, HA expression, and proline uptake levels in HCH cells. The cells were treated with or without 1 μg/mL AMS for 24 h. The relative level of (A) glucosamine uptake, (B) HA secreted into culture medium, and (C) proline uptake were analyzed in AMS treated HCH cells. The results are presented as the mean ± SD (n = 3). Statistical analysis was performed by one-way ANOVA and followed by Scheffe’s post-hoc test using SPSS software. *p < 0.05 and ***p < 0.001 relatives to untreated normal control group, and #p < 0.05 and ##p < 0.01 relative to AMS-treated group.
AMS restored the IL-1b-reduced collagen II level in human chondrocytes
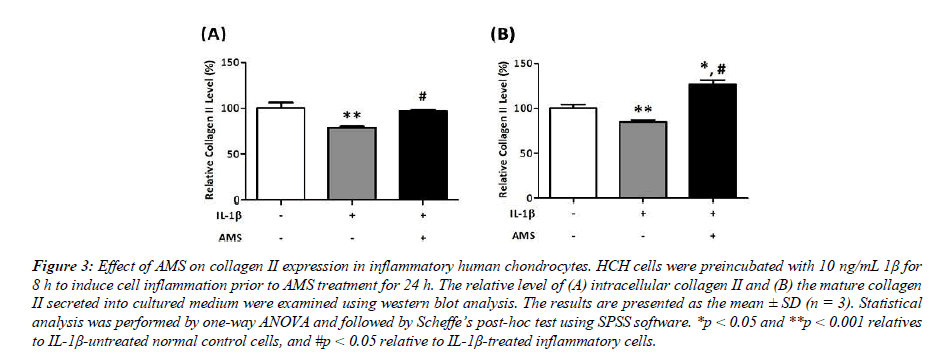
In this study, both of the intracellular and secreted mature collagen II expression levels were examined in IL-1β- induced inflammatory HCH cells. As shown in Figure 3, the collagen II levels are significantly decreased in IL-1β treated cells (78.7% of the intracellular and 84.8% of the secreted form, respectively, p<0.01 vs control cells). In IL-1β- induced inflammatory cells, AMS significantly restored the intracellular collagen II expression by 23% (from 78.7% to 97.1%, #p<0.05 vs IL-1β-treated cells). In a similar manner, AMS conferred a significant 49% increase in mature collagen II level secreted into ECM (from 84.8% to 126.7%, p<0.05 vs IL-1β -treated cells). These results indicate that AMS effectively ameliorates the IL-1-induced inflammatory damage in HCH cells by enhancing collagen II synthesis which may potentially help to rebuild the cartilage tissues.
Figure 3: Effect of AMS on collagen II expression in inflammatory human chondrocytes. HCH cells were preincubated with 10 ng/mL 1β for 8 h to induce cell inflammation prior to AMS treatment for 24 h. The relative level of (A) intracellular collagen II and (B) the mature collagen II secreted into cultured medium were examined using western blot analysis. The results are presented as the mean ± SD (n = 3). Statistical analysis was performed by one-way ANOVA and followed by Scheffe’s post-hoc test using SPSS software. *p < 0.05 and **p < 0.001 relatives to IL-1β-untreated normal control cells, and #p < 0.05 relative to IL-1β-treated inflammatory cells.
Effect of AMS on MMP-1 and MMP-13 expression in human chondrocytes
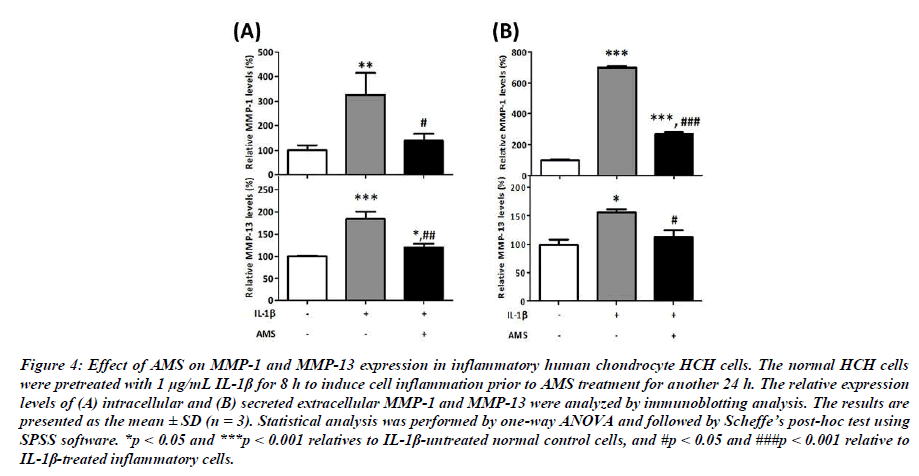
To understand the protective effect of AMS on chondrocytes, we next examined the intracellular and extracellular MMP-1 and MMP-13 expressed in normal control or IL-1β-induced inflammatory HCH cells. Compare with normal control cells, results shown in Figure 4 indicate that inflammatory cytokine IL-1β significantly induced the intracellular and extracellular MMP-1 and MMP-13 expression in these cells. As shown in Figure 4, the presence of AMS significantly suppressed IL- 1-induced intracellular MMP-1 level by 57% (from 325% to 140%, p<0.05 vs IL-1β-treated cells) and MMP-13 level by 35% (from 184% to 120%, p<0.01 vs IL-1β-treated cells) back to normal range. Similarly, the results shown in Figure 4 indicate that AMS also significantly inhibited the IL-1β- induced secreted extracellular MMP-1 level by 62% (from 702% to 270%, p<0.001 vs IL-1β-treated cells) and MMP- 13 level by 28% (from 156% to 113%, p<0,05 vs IL-1β- treated cells) in HCH cells. These results indicate that the inflammation-induced MMPs expression can be significantly surpressed by AMS, thus protect joint cartilage from inflammation challenges.
Figure 4: Effect of AMS on MMP-1 and MMP-13 expression in inflammatory human chondrocyte HCH cells. The normal HCH cells were pretreated with 1 μg/mL IL-1β for 8 h to induce cell inflammation prior to AMS treatment for another 24 h. The relative expression levels of (A) intracellular and (B) secreted extracellular MMP-1 and MMP-13 were analyzed by immunoblotting analysis. The results are presented as the mean ± SD (n = 3). Statistical analysis was performed by one-way ANOVA and followed by Scheffe’s post-hoc test using SPSS software. *p < 0.05 and ***p < 0.001 relatives to IL-1β-untreated normal control cells, and #p < 0.05 and ###p < 0.001 relative to IL-1β-treated inflammatory cells.
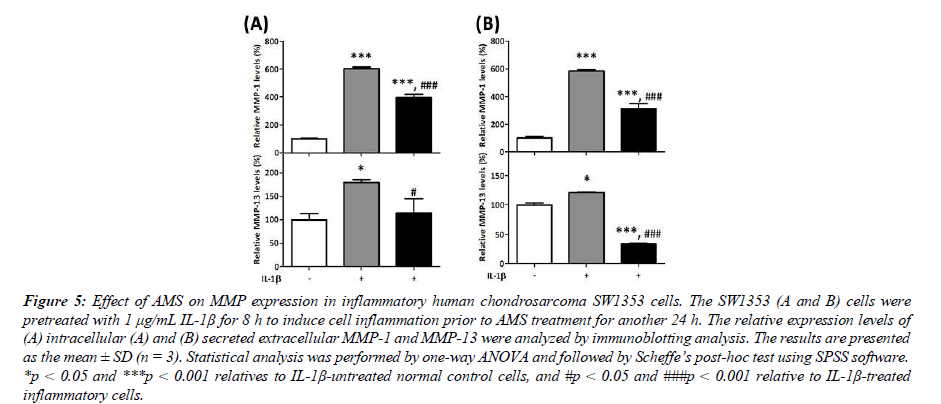
Effect of AMS on MMP-1 and MMP-13 expression in human chondrosarcoma cells
The chondrosarcoma SW1353 cells were employed to further evaluate the protective effect of AMS on human cartilage tissue in this study. The effect of AMS on the intracellular and extracellular MMP-1 and MMP-13 expressed in normal control or IL-1β-induced inflammatory SW1353 cells were examined. Compare with IL-1β-untreated control cells, results shown in Figure 5 indicate that inflammatory cytokine IL-1β significantly induced the intracellular and extracellular MMP- 1 and MMP-13 expression similarly in this cell model. Results shown in Figure 5 indicate that AMS significantly inhibited IL-1β-induced intracellular MMP-1 level by 34% (from 604% to 397%, p<0.001 vs IL-1β-treated cells) and MMP-13 level by 36% (from 180% to 115%, p<0.05 vs IL-1β-treated cells). In a similar pattern, the results shown in Figure 5 indicate that AMS also significantly inhibited the IL-1β-induced secreted extracellular MMP-1 level by 47% (from 584% to 312%, p<0.001 vs IL-1β-treated cells) and MMP-13 level by 72% (from 122% to 34%, p<0.001 vs IL-1β-treated cells) in SW1353 cells. These results indicate that the protective effect of AMS against inflammatory challenges is general property and not limited to a specific cell type.
Figure 5:Effect of AMS on MMP expression in inflammatory human chondrosarcoma SW1353 cells. The SW1353 (A and B) cells were pretreated with 1 μg/mL IL-1β for 8 h to induce cell inflammation prior to AMS treatment for another 24 h. The relative expression levels of (A) intracellular (A) and (B) secreted extracellular MMP-1 and MMP-13 were analyzed by immunoblotting analysis. The results are presented as the mean ± SD (n = 3). Statistical analysis was performed by one-way ANOVA and followed by Scheffe’s post-hoc test using SPSS software. *p < 0.05 and ***p < 0.001 relatives to IL-1β-untreated normal control cells, and #p < 0.05 and ###p < 0.001 relative to IL-1β-treated inflammatory cells.
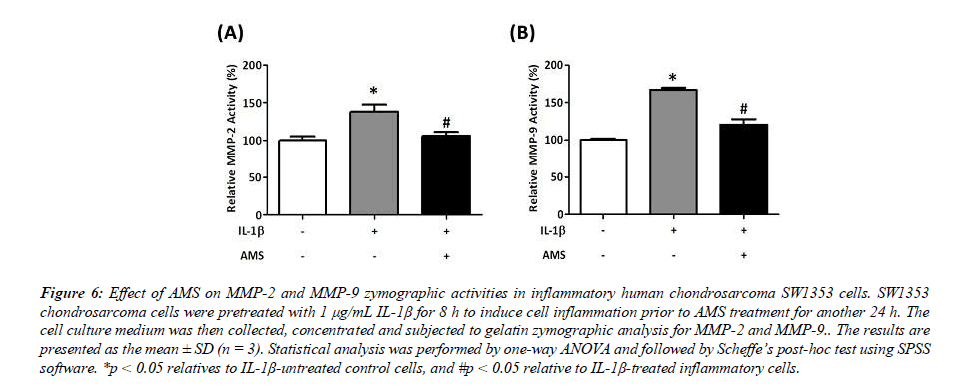
AMS attenuates MMP-2 and MMP-9 zymographic activities in inflammatory human chondrosarcoma cells
MMPs are generally secreted as inactive proenzyme and proteolytically activated extracellularly by a range of proteases which is tightly regulated by appropriate physiological conditions. In this study, the protective effect of AMS on inflammatory cytokine IL-1β-induced increased in MMP- 2 and MMP-9 activities were examined in chondrosarcoma SW1353 cells. Compare with IL-1β-untreated control cells, results shown in Figure 6 indicate that inflammatory cytokine IL-1β significantly induced the Proteolytic activities of MMP- 2 and MMP-9 in these cells (137.6% for MMP-2 and 166.8% for MMP-9, respectively, p<0.05 vs control cells). Results shown in Figure 6 also indicate that AMS significantly suppressed IL-1β-induced MMP-2 activity by 23% (panel 6A, from 137.6% to 105.8%, p<0.05 vs IL-1β-treated cells) and MMP-9 activity by 28% (panel 6B, from 166.8% to 120.5%, p<0.05 vs IL-1β-treated cells). These results demonstrate that the joint cartilage damage induced by inflammatory cytokines can be significantly ameliorated by AMS in human chondrocytes.
Figure 6:Effect of AMS on MMP-2 and MMP-9 zymographic activities in inflammatory human chondrosarcoma SW1353 cells. SW1353 chondrosarcoma cells were pretreated with 1 μg/mL IL-1β for 8 h to induce cell inflammation prior to AMS treatment for another 24 h. The cell culture medium was then collected, concentrated and subjected to gelatin zymographic analysis for MMP-2 and MMP-9.. The results are presented as the mean ± SD (n = 3). Statistical analysis was performed by one-way ANOVA and followed by Scheffe’s post-hoc test using SPSS software. *p < 0.05 relatives to IL-1β-untreated control cells, and #p < 0.05 relative to IL-1β-treated inflammatory cells.
Discussion
This study is designed to investigate the effect of AMS, an herbal preparation of highly enriched saponins from Astraglus membranceus, on protection of chondrocytes. The cycloartane type of triterpene saponins fraction contains a mixture of astragalosides with a broad range of pharmacological activities, including anti-inflammatory, anti-aging, wound healing, immunomodulatory and anti-cancer activities [9,10]. Our results showed that there is no apparent cytotoxicity of AMS for the concentration range from 0.1 to 50 μg/mL toward both of primary HCH and chondrosarcoma SW1353 cells, indicates the pharmacological safety of AMS (Figure 1). In this study, we showed that AMS displays prominent effect on stimulation of glucosamine uptake, HA level, and proline uptake in primary HCH cells (Figure 2). Using the in vitro OA model, AMS significantly increases chondrocyte specific collagen II expression in IL-1β-induced inflammatory chondrocytes (Figure 3). Moreover, AMS was shown to ameliorate inflammatory damages of chondrocytes by suppression of MMP-1 and MMP-13 expression and Proteolytic activities of MMP-2 and MMP-9 (Figure 4, 5, and 6). Collectively, our results demonstrate that AMS displays a potent protective effect on joint cartilage from inflammatory challenge by inhibition the MMPs expression and Proteolytic activities and also furnish the cartilage ECM construction by enhancing collagen II and HA expression levels.
The cartilage ECM plays a crucial role in maintaining the integrity and function of the cartilage tissues. Glucosamine is the major building block for glycosaminoglycans (GCGs), such as HA, which is the essential component of the ECM in cartilage and synovial fluid of joints (13). It has also been shown that uptake of glucosamine by chondrocytes plays an important role in cartilage health. In healthy joints, HA is synthesized and secreted by chondrocytes and synoviocytes, and its concentration is well-balanced in between its production and degradation [14]. Glucosamine has been shown to have anti-inflammatory and chondroprotective effects as shown by in vitro and in vivo studies [15,16]. In human clinical trials on glucosamine supplementation showed positive effects to joint pain and function [17]. In addition, study also indicate that glucosamine increases HA production in human OA synovium tissue [18]. In our study, AMS was shown to facilitate glucosamine uptake and enhance HA production in chondrocytes (Figure 2). Considering that glucosamine is the primary building block of HA, we demonstrate here that AMS plays a crucial role in facilitating ECM and cartilage formation.
In addition to glucosamine uptake, the effect of AMS on proline uptake was also assessed in HCH cells. In human cells, proline contributes as the primary building block for collagen, which is vital for cartilage function and health. Thus, measuring proline uptake can reflect the degree of collagen synthesis in these cells. Here, we showed that AMS significantly increased proline uptake in HCH cells (Figure 2), indicate the critical role of AMS in contributing to collagen synthesis. The intracellular proline level is important for chondrocytes in maintenance of cartilage structure. Previous study demonstrated that inadequate proline uptake in chondrocytes is associated with a decreased collagen synthesis and impaired cartilage matrix production, resulting in development and progression of OA [19-21]. Thus, here we showed that AMS is capable of enhance proline uptake, which can support collagen synthesis and help to maintain cartilage normal function.
In AC, collagen is the most abundant protein in the ECM of cartilage. It provides mechanical strength and stability to the tissue and is crucial for maintaining its structural integrity. Among the many collagen subtypes, collagen II is the major collagen subtype in articular cartilage tissue. The destruction of cartilage is the hallmark of OA and the degradation of type II collagen is the pivotal event that determines progression of OA disease. Previous studies demonstrated by that supplementation of type II collagen can effectively ameliorate inflammatory responses and AC damage in an in vivo OA model [22]. Upon inflammation, chondrocytes respond to inflammatory cytokines by producing matrix-degrading enzymes, such as MMPs, that target collagen II. Damaged collagen fibre network and reduced synthesis of collagen II leads to cartilage degradation. Therapeutic strategy that focuses on enhanced collagen II synthesis should be helpful to cartilage integrity and slow the progression of OA. Recent studies found that the agents such as glucosamine sulphate and chondroitin sulphate can increase proline uptake and collagen synthesis in chondrocytes, which is important for maintaining cartilage health [23]. In the present study, IL-1β was shown to significantly decreased collagen II level in HCH cells; nevertheless, the expression level of collagen II is completely restored in the HCH cells when co-incubated with AMS (Figure 3). These findings indicate that AMS exhibits a significant beneficial effect and can successfully fight against inflammation in chondrocytes.
MMPs are a family of metal-dependent proteinases which are highly expressed in cartilage. Studies indicate that increased MMPs expression in inflammation plays a central role in the progression of OA [24]. Among the MMP ribozymes, MMP- 1 and MMP-13 are considered as the key enzymes in OA progression because they are rate-limiting in the process of collagen II and ECM degradation [25,26]. MMP-1 is produced primarily in synovial cells that line joints, and MMP-13 is a product of chondrocyte that resides in cartilage. The depletion of the MMP-13 gene in mice has been shown to inhibit the progression of OA, demonstrating the crucial role of MMP- 13 in cartilage erosion [27]. Recent studies also indicate that natural compounds such as curcumin can decrease MMP-13 production in inflammatory human chondrocytes, leading to increased collagen II synthesis and cartilage protection [28].
In addition to MMP-1 and MMP-13, studies also indicate that MMP-2 and MMP-9 were highly expressed and active in areas synovial inflammation and cartilage damage [29]. The findings indicate the involvement of MMP-2 and MMP-9 activities in pathogenesis and progression of OA. The current study showed that both of intracellular and secreted MMP- 1 and MMP-13 were greatly increased in inflammatory in HCH and SW1353 cells. Nevertheless, co-incubation of AMS significantly inhibits the IL-1β-induced expression of MMP-1 and MMP-13, returning their levels to normal in HCH chondrocytes (Figure 4) and chondrosarcoma cells (Figure 5). Moreover, AMS also significantly suppressed the IL-1β-induced Proteolytic activities of MMP-2 and MMP-
9 in chondrosarcoma cells, bringing their levels back to normal range (Figure 6). All of this information highlights the potential efficacy of AMS in ameliorating inflammation- induced damages in chondrocytes and chondrosarcoma cells.
Astragalus is a top-grade traditional Chinese medicine that has been shown to be safe, no apparent toxicity, and used for centuries to treat various health conditions. Previously studies have shown the therapeutic efficacy of astragalus or astragalosides IV in treatment of OA and cartilage damages [30-32]. Nevertheless, further investigation is required as the detailed mechanisms and bioactive components are largely unknown. In the current study, we showed that AMS exerts potent chondroprotective effects by inhibition of MMP production and promotion collagen II synthesis in IL-1- induced inflammatory chondrocytes. Our results provide further evidence to show the therapeutic potential of AMS in the treatment of OA and cartilage damages. Nevertheless, additional research is necessary to get a better understanding of its effectiveness and potential applications in this field.
Conclusion
Currently, the treatment of knee and hip OA primarily focused on pharmacological interventions, such as Non- Steroidal Anti-Inflammatory Drugs (NSAIDs), analgesics, and intra-articular corticosteroids, which alleviate pain and inflammation. However, these drugs are associated with long- term side effects, and prosthetic surgery is often the ultimate solution for end-stage OA. To address these challenges, there is a need for the development of novel medications that offer greater efficacy and safer therapeutic options. The current study highlights the potential of AMS in preventing cartilage degeneration by promoting HA and collagen II synthesis, as well as down regulating MMPs expression and activation in inflammatory chondrocytes. In conclusion, we demonstrate that AMS possesses chondroprotective activity and could serve as potential therapeutic agent inflammatory cartilage damage in joints.
Acknowledgements
This work was supported by grants from the Ministry of National Defense (MAB-109-055, MAB-110-118, and MND- MAB-111092 to T-C.C.) and Cheng-Hsin General Hospital (CH-NDMC-106-15 and CH-NDMC-107-2 to T-C. C.),
Taipei, Taiwan, ROC. We are also grateful to Nuliv Science, Inc., for generous assistance in this work.
Author Contributions
CH. O., IC T., SF. H., HW. Y., WL. C. and TC. C. participated in the concept of the study and the experimental design. IC T. and WL. C. isolated and characterized the AMS. CH. O., IC T., SF. H., and HW. Y. were involved in laboratory experiments and data analysis. TC. C. wrote the manuscript.
Conflict of Interest
The authors declare no conflict of interest.
References
- Martel-Pelletier J, Barr AJ, Cicuttini FM, et al. Osteoarthritis. Nat Rev Dis Primers. 16072.
- Sarzi-Puttini P, Cimmino MA, Scarpa R, et al. Osteoarthritis: an overview of the disease and its treatment strategies. Semin Arthritis Rheum. 2005;35(1):1-10.
- Sophia Fox AJ, Bedi A, et al. The basic science of articular cartilage: Structure, composition, and function. Sports health. 2009;1(6):461-8.
- Eyre DR. The collagens of articular cartilage. Semin Arthritis Rheum. 1991;21(3):2-11.
- Buckland J. Targeting cartilage erosion in OA. Nat Rev Rheumatol. 2010;6(2):64.
- Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745-59
- Daheshia M, Yao JQ. The interleukin 1β pathway in the pathogenesis of osteoarthritis. J Rheumatol. 2008;35(12):2306-12.
- Kunisch E, Kinne RW, Alsalameh RJ, et al. Pro‐ inflammatory IL‐1beta and/or TNF‐alpha up‐regulate matrix metalloproteases‐1 and‐3 m RNA in chondrocyte subpopulations potentially pathogenic in osteoarthritis: in situ hybridization studies on a single cell level. Int J Rheum Dis. 2016;19(6):557-66.
- Zheng Y, Ren W, Zhang L, et al. A review of the pharmacological action of Astragalus polysaccharide. Front Pharmacol. 2020;11:349.
- Guo Z, Lou Y, Kong M, et al. A systematic review of phytochemistry, pharmacology andpharmacokinetics on astragali radix: Implications for astragali radix as a personalized medicine. Int J Mol Sci. 2019;20(6):1463.
- Fernandez-Patron C, Radomski MW, Davidge ST. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ Res. 1999;85(10):906-11.
- Nicklin PL, Irwin WJ, Hassan IF, et al. Proline uptake by monolayers of human intestinal absorptive (Caco- 2) cells in vitro. Biochim Biophys Acta Biomembr. 1992;1104(2):283-92.
- Uitterlinden EJ, Koevoet JL, Verkoelen CF, et al. Glucosamine increases hyaluronic acid production in human osteoarthritic synovium explants. BMC Musculoskelet Disord. 2008;9(1):1-7.
- Knudson W, Ishizuka S, Terabe K, et al. The pericellular hyaluronan of articular chondrocytes. Matrix Biol. 2019;78:32-46.
- Henrotin Y, Lambert C. Chondroitin and glucosamine in the management of osteoarthritis: An update. Curr Rheumatol Rep. 2013;15:1-9.
- Hochberg MC. Structure-modifying effects of chondroitin sulfate in knee osteoarthritis: an updated meta-analysis of randomized placebo-controlled trials of 2-year duration. Osteoarthr Cartil. 2010;18:S28-31.
- Lubis AM, Siagian C, Wonggokusuma E, et al. Comparison of glucosamine-chondroitin sulfate with and without methylsulfonylmethane in grade I-II knee osteoarthritis: a double blind randomized controlled trial. Acta Med Indones. 2017;49(2):105-11.
- Bruyere O, Reginster JY. Glucosamine and chondroitin sulfate as therapeutic agents for knee and hip osteoarthritis. Drugs & aging. 2007;24:573-80.
- Karna E, Szoka L, Huynh TY, et al. Proline-dependent regulation of collagen metabolism. Cell Mol Life Sci. 2020;77:1911-8.
- Nakatani S, Mano H, Sampei C, et al. Chondroprotective effect of the bioactive peptide prolyl-hydroxyproline in mouse articular cartilage in vitro and in vivo. Osteoarthr Cartil. 2009;17(12):1620-7.
- Videman T, Eronen I, Candolin T. [3H] prolineincorporation and hydroxyproline concentration in articular cartilage during the development of osteoarthritis caused by immobilization. A study in vivo with rabbits. Biochem J 1981;200(2):435-40.
- Orhan C, Juturu V, Sahin E, et al. Undenatured type II collagen ameliorates inflammatory responses and articular cartilage damage in the rat model of osteoarthritis.
- Igarashi M, Sakamoto K, Nagaoka I. Effect of glucosamine on expression of type II collagen, matrix metalloproteinase and sirtuin genes in a human chondrocyte cell line. Int J Mol Med. 2017;39(2):472-8.
- Cabral-Pacheco GA, Garza-Veloz I, Castruita-De la Rosa C, et al. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int J Mol Sci. 2020;21(24):9739.
- Gho WG, Choi Y, Park KH, et al. Expression of collagenases (matrix metalloproteinase-1, 8, 13) and tissue inhibitor of metalloproteinase-1 of retrodiscal tissue in temporomandibular joint disorder patients. J Korean Assoc Oral Maxillofac Surg. 2018;44(3):120.
- Wang M, Sampson ER, Jin H, et al. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther. 2013;15(1):1-1.
- Little CB, Barai A, Burkhardt D, et al. Matrix metalloproteinase 13–deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology. 2009;60(12):3723-33.
- Wang J, Ma J, Gu JH, et al. Regulation of type II collagen, matrix metalloproteinase-13 and cell proliferation by interleukin-1β is mediated by curcumin via inhibition of NF-κB signaling in rat chondrocytes. Mol Med Rep. 2017;16(2):1837-45.
- Zeng GQ, Chen AB, Li W, et al. High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genet Mol Res. 2015;14(4):14811-22.
- Maresca M, Micheli L, Cinci L, et al. Pain relieving and protective effects of Astragalus hydroalcoholic extract in rat arthritis models. J Pharm Pharmacol. 2017;69(12):1858- 70.
- Wang B, Chen MZ. Astragaloside IV possesses antiarthritic effect by preventing interleukin 1β-induced joint inflammation and cartilage damage. Arch Pharm Res. 2014;37:793-802.
- Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis: A review. JAMA. 2021;325(6):568-78.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Front Vet Sci. 2021;8:617789.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref