Research Article - Biomedical Research (2017) Volume 28, Issue 5
Therapeutic effects and toxic side reactions of capecitabine combined with a modified prescription of Fuzheng Jiedusan (resistance strengthening and detoxification granules) on advanced gastric cancer
Shengli Zhou*, Xiulai Zhang and Zhenya SongDepartment of General Medicine, International Health Center, the Second Affiliated Hospital, Zhejiang University School of Medicine, No. 88 Jiefang Road, Hangzhou, Zhejiang Province, PR China
- *Corresponding Author:
- Shengli Zhou
Department of General Medicine, International Health Center
The Second Affiliated Hospital, Zhejiang University School of Medicine
PR China
Accepted date: January 02, 2016
Abstract
Objective: To evaluate the therapeutic effects and toxic side reactions of capecitabine combined with a modified prescription of Fuzheng Jiedusan on advanced gastric cancer.
Methods: A total of 145 patients were divided into a research group (n=69) and a control group (n=76) according to hospital admission sequence, and treated by capecitabine combined with a modified prescription of Fuzheng Jiedusan and only capecitabine respectively to compare the short-term effects (tumor changes), quality of life, long-term effects and incidence of toxic side effects.
Results: The therapeutic response rate and disease control rate of research group were 30.4% and 68.1% respectively. The quality of life of 59.5% (41 patients) of research group was improved. After treatment, research group had a significantly higher KPS score than that before treatment and that of control group (P<0.05). The time to progression and survival time of research group were 2-19.5 months and 4.5-24 months respectively. Sixty patients in research group survived, with a significantly higher survival rate (87.0%) than that of control group (59 patients, 77.9%) (P<0.05). The incidence rates of hand-foot syndrome, nausea and vomiting, diarrhoea, abdominal pain, liver injury and neurotoxicity in research group were 21.7%, 2.9%, 11.6%, 7.2%, 1.4% and 17.4% respectively, all of which were significantly lower than those of control group (43.4%, 22.3%, 25%, 21.1%, 11.8% and 36.8%).
Conclusion: Capecitabine combined with a modified prescription of Fuzheng Jiedusan had good therapeutic effects on advanced gastric cancer, with few toxic side effects and improved quality of life.
Keywords
Capecitabine, Fuzheng Jiedusan, Advanced gastric cancer
Introduction
With social development and changes in lifestyle, gastrointestinal tumors, especially gastric cancer, have seriously threatened human life and health [1]. As an oral cytotoxic preparation of fluorouracil, capecitabine can be conveniently taken and exert satisfactory therapeutic effects on gastrointestinal tumors [2]. However, capecitabine may cause hand-foot syndrome, stomatitis, nausea, vomiting and other adverse reactions in clinical practice, thus severely affects the quality of life of patients [3]. Tang et al. reported that combining chemotherapy with a Traditional Chinese Medicine (TCM) drug reduced toxic side effects and relieved the symptoms of patients during tumor treatment [4]. Therefore, gastric cancer may be better treated by combining capecitabine with a proper TCM drug to alleviate the side effects simultaneously.
Fuzheng Jiedusan, as a TCM composite formula, mainly comprises Radix Isatidis, Radix Astragali and Epimedium brevicornum Maxim. It has been used to treat hepatocellular carcinoma [5]. Yin et al. found that Fuzheng Jiedusan prolonged the survival and inhibited the tumor metastasis of BALB/c athymic mice [6]. This formula can eliminate toxins and regulate blood circulation. Besides, Yu et al. reported that Fuzheng Jiedusan had a number of health benefits through modulation of the immunity [7].
Thereby motivated, we herein combined capecitabine with a modified prescription of Fuzheng Jiedusan to treat patients with advanced gastric cancer and poor tolerance, and their quality of life was improved after treatment.
Materials and Methods
Basic information
The subjects of this study were 145 patients with gastric cancer diagnosed through operations, gastroscopic and pathological examinations in our hospital from January 2011 to January 2014, and all of them had measurable tumor lesions. They were aged 45 to 85, and the average was (62.3 ± 6.7) years old. There were 75 males and 70 females. According to TNM classification, all of them were Stage VI patients. As to Karnofsky Performance Status (KPS) scores, 9 patients had scores of lower than 60, 70 had scores from 61 to 70, 47 had scores from 71 to 80, and 11 had scores from 81 to 90. The expected survival time was longer than 3 months. No chemotherapy contraindications were indicated in the blood routine examinations, electrocardiograms or hepatic and renal function examinations. Based on hospital admission sequence, they were divided into a control group (76 patients) and a research group (69 patients), and treated by capecitabine only and capecitabine combined with a modified prescription of Fuzheng Jiedusan and respectively. The two groups had similar gender ratio, age and course of disease (P>0.05). In addition, all patients and their families approved this study and signed the informed consent.
Research methods
All patients orally took 1000 mg/m2 capecitabine (product name: Xeloda; purchased from Shanghai Roche Pharmaceuticals Ltd.) in 30 min after dinner twice per day for continuously 14 days, and then the administration was suspended for 7 days. In other words, 21 days were considered as a dosing period. Each patient orally took capecitabine for 2 periods at least. Blood routine tests as well as hepatic and renal function examinations were conducted before and after the cessation of chemotherapy, and the therapeutic effects were evaluated.
TCM treatment regimen: Patients had orally taken Fuzheng Jiedusan twice per day till the cessation of chemotherapy course since they orally took capecitabine. Appropriate ingredients should be added or removed based on the toxic and side effects of capecitabine as well as the patients’ physiques. Specifically, 10 g of Corydalis yanhusuo, 10 g of Curcuma sichuanensis and 10 g of Aspongopus chinensis were added for patients with abdominal pain; Radix Rehmanniae Preparata was removed while 10 g of stir-fried Rhizoma Dioscoreae, 10 g of A. chinensis and 10 g of Poria cocos were added for diarrheal patients; 10 g of ginger-processed Pinellia ternata, 10 g of Pericarpium Citri Reticulatae, 6 g of nutmeg and 6 g of Fructus Amomi were added for nauseated patients; 15 g of Rhizoma Alismatis, 6 g of Ramulus Cinnamomi and 15 g of Pericarpium Arecae were added for patients with peritoneal effusion; 15 g of Radix Ophiopogonis, 15 g of Radix Scrophulariae and 15 g of Fructus Ligustri Lucidi were added for patients with deficiency of yin, gastric discomfort and red tongues with few coatings; 12 g of Fructus Psoraleae, 12 g of Herba Epimedii and 12 g of Fructus Evodiae were added for patients with deficiency of yang and intolerance of cold, vomiting, as well as deep and slight pulses; 20 g of Cortex Dictamni, 20 g of Radix Sophorae Flavescentis, 20 g of Zaocys Dhumnades and 20 g of Caulis Spatholobi were added for patients with severe hand-foot syndrome.
Investigation indices
Short-term therapeutic effect (tumor changes), quality of life, long-term therapeutic effect and toxic side reactions were taken as the indices to evaluate the treatment outcomes. (1) The short-term therapeutic effect was evaluated according to tumor changes. After the cessation of chemotherapy, MRI or CT re-examination was conducted to observe the size of tumor according to the RECIST standard [8]. Such effects were classified into Progressive Disease (PD), Stable Disease (SD), Partial Response (PR) and Complete Response (CR); effective rate: PR+CR; disease control rate: PR+CR+SD. (2) According to KPS evaluation standards [9], the quality of life was classified into improved (difference: higher than 10 points), stable (difference: 10 to -10 points) and lowered (difference: less than -10 points). (3) Long-term therapeutic effects: The time to progression and survival time were observed; the survival time was from the beginning of chemotherapy to death; the survival rates of the two groups within 1 year were compared. (4) Toxic side effects: Based on the Grading Standards for Acute and Subacute Toxic Reactions of Anti- Cancer Drugs [10] constituted by WHO, such effects were classified into grade 0-IV.
Statistical analysis
SPSS17.0 was used for data analysis. The χ2 test was taken for the comparison of counting data, and the t test was carried out for the comparison of measurement data. P<0.05 was considered statistically significant.
Results
Comparison of short-term therapeutic effects
The therapeutic response rate and disease control rate were 30.4% and 68.1% respectively for the research group and 25% and 51.3% respectively for the control group. The chi-square test indicated no significant difference between two groups (P>0.05) (Table 1).
| Group | n | CR | PR | SD | PD | Response rate (%) | Disease control rate (%) |
|---|---|---|---|---|---|---|---|
| Research group | 69 | 1 | 20 | 26 | 22 | (21) 30.4 | (47) 68.1 |
| Control group | 76 | 2 | 17 | 20 | 37 | (19) 25 | (39) 51.3 |
| χ2 | 2.13 | 2.01 | |||||
| P | 0.051 | 0.057 |
Table 1: Comparison of short-term therapeutic effects.
Comparison of quality of life
The quality of life of 59.5% (41) patients in the research group was improved. As indicated by the chi-square test, after treatment, the KPS score of the research group was significantly higher than that before treatment and that of the control group (P<0.05) (Table 2).
| Group | n | Before treatment | Treatment |
|---|---|---|---|
| Research group | 69 | 62.1±5.1 | 83.5±5.9*# |
| Control group | 76 | 61.2±5.4 | 58.9±4.8 |
| *P<0.05 for intra-group comparison; #P<0.05 for inter-group comparison. | |||
Table 2: Comparison of KPS scores for quality of life of two groups.
Comparison of long-term therapeutic effects
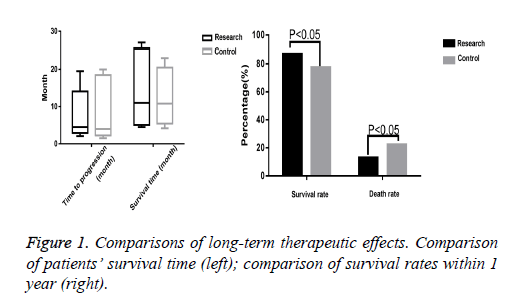
The time to progression and survival time of the research group were 2 to 19.5 months and 4.5 to 24 months respectively, and only 5 patients survived. The control group’s time to progression and survival time were 1.5 to 20 months and 4.2 to 23 months respectively, and only 3 patients survived. The t-test indicated no significant differences between the two groups (P>0.05). Besides, 60 patients survived in the research group, with a significantly higher survival rate (87.0%) than that of the control group (59 patients, 77.9%) (P<0.05) (Figure 1).
Comparison of adverse reactions
The incidence rates of hand-foot syndrome, nausea and vomiting, diarrhoea, abdominal pain, liver injury and neurotoxicity in the research group were 21.7%, 2.9%, 11.6%, 7.2%, 1.4% and 17.4% respectively, all of which were significantly lower than those of the control group (43.4%, 22.3%, 25%, 21.1%, 11.8% and 36.8%) (P<0.05) (Table 3).
| Toxic and side reactions | Control group (76 patients) | Research group (69 patients) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 0 | Grade I | Grade II | Grade III | Grade IV | Incidence rate (%) | Grade 0 | Grade I | Grade II | Grade III | Grade IV | Incidence rate (%) | |
| Hand-foot syndrome | 43 | 26 | 7 | 0 | 0 | 43.4 | 54 | 12 | 3 | 0 | 0 | 21.7* |
| Nausea and vomiting | 59 | 13 | 4 | 0 | 0 | 22.3 | 67 | 2 | 0 | 0 | 0 | 2.9* |
| Diarrhoea | 57 | 11 | 8 | 0 | 0 | 25 | 61 | 8 | 0 | 0 | 0 | 11.6* |
| Abdominal pain | 60 | 12 | 4 | 0 | 0 | 21.1 | 64 | 5 | 0 | 0 | 0 | 7.2* |
| Liver and kidney damage | 67 | 5 | 4 | 0 | 0 | 11.8 | 68 | 1 | 0 | 0 | 0 | 1.4* |
| Electrocardiogram | 66 | 6 | 4 | 0 | 0 | 13.1 | 61 | 6 | 2 | 0 | 0 | 11.6 |
| Neurotoxicity | 48 | 22 | 6 | 4 | 0 | 36.8 | 57 | 8 | 4 | 0 | 0 | 17.4* |
| Compared with the control group, *P<0.05. | ||||||||||||
Table 3: Comparison of incidences of toxic and side reactions.
Discussion
With the incidence rate ranking fourth and the death rate ranking second, gastric cancer is the most common malignant tumor and 360,000 people die of it in our country every year [11]. Therefore, it is of great significance to develop effective therapeutic methods for advanced gastric cancer to improve the quality of life and survival [12]. Capecitabine is a fluorouracil anti-cancer drug, and its single oral therapeutic effect notably surpasses intravenous injection of 5-fluorouracil. The drug concentration in tumor tissue is obviously higher than that in normal tissue. Besides, capecitabine has good anti-cancer effects on all drug-resistant cell lines. Okines et al. reported that the effective rate of treating advanced gastric cancer by orally taking capecitabine reached 24% [13], so patients can choose to orally take capecitabine when they are unwilling to accept combined chemotherapy or have poor tolerance. The most notable adverse reaction of taking capecitabine orally is hand-foot syndrome. Azuma et al. reported that the incidence rate of capecitabine-induced hand-foot syndrome was 44.4% [14]. Zhu et al. found that capecitabine-induced adverse reactions were significantly related to its oral dosages [15]: When 2500 mg of capecitabine was taken orally, the incidence rate of hand-foot syndrome was 100%; however, when 100% of capecitabine was taken orally, such rate was decreased to 77.08%. Thus, to avoid a significant adverse reaction, it is crucial to choose a proper dosage of capecitabine.
Moreover, TCM is also a well-documented therapeutic method for tumors and has remarkable advantages in overcoming adverse reactions of chemotherapeutics [16]. TCM involves the asthenia of pathogens and vital qi for the recognition for tumor pathogenesis [17]. In this study, patients with advanced gastric cancer were included. The stomach is the foundation of acquired constitution. Therefore, in case of pathogeneses such as weakness of the spleen and the stomach, mechanism clogged by toxicity and insufficiency of vital energy and blood, Radix Astragali, Atractylodes macrocephala Koidz and Codonopsis pilosula can be used to boost the vitality [18], Radix Paeoniae Alba, Polygonatum kingianum and Radix Rehmanniae Preparata can be used to nourish blood supply [19], Salvia chinensis, Curcuma zedoary and raw oyster shell can be employed to invigorate blood circulation, and Scutellaria barbata and edible tulip can be utilized for disinfection [20]. Modifying ingredients according to symptoms helps guide the “qi” downward and control nausea and vomiting. Additionally, it can nourish yin, tonify yang, strengthen the body resistance, relieve internal heat or fever and alleviate toxic side reactions.
In this study, the therapeutic effect of combining capecitabine and TCM drugs on gastric cancer was equal to that of individual capecitabine, and there was no significant difference between their survival times. However, the quality of life of the research group was notably improved and the incidence rates of toxic side reactions were lower than those of the control group. Hence, combining capecitabine with Fuzheng Jiedusan, a TCM drug, indeed reduced the toxic side reactions of drugs, accompanied by improved survival quality and disease control rate for the advanced gastric cancer patients with poor resistance and physical power. This study, which dialectically combined the syndrome differentiation and disease differentiation by modifying the prescription based on patients’ toxic and side reactions, can be regarded as an effective exploration in targeted treatment of tumor through combination of TCM and Western medicine. Similarly, Jin et al. treated advanced digestive tract cancers by combining qi-replenishing and blood circulation-activating drugs with chemotherapy preparations, achieving favourable effects [21].
Conclusion
Combining capecitabine with a modified prescription of Fuzheng Jiedusan effectively enhanced the clinical benefit response of patients, and significantly mitigated the toxic side reactions of capecitabine such as hand-foot syndrome and vomiting, indicating the combined treatment using Chinese herb was clearly capable of toxicity reduction and efficacy enhancement. Thus, this treatment strategy is worthy of promotion due to remarkable therapeutic effects on advanced digestive tract tumors, few toxic side reactions, and significantly improved quality of life. However, the sample size needs to be enlarged to further verify the advantages of combining TCM with Western medicine in treating gastric cancer.
References
- Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014; 23: 700-713.
- Park SH, Sohn TS, Lee J, Lim do H, Hong ME, Kim KM, Sohn I, Jung SH, Choi MG, Lee JH, Bae JM, Kim S, Kim ST, Park JO, Park YS, Lim HY, Kang WK. Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: Final report of the adjuvant chemoradiotherapy in stomach tumors trial, including survival and subset analyses. J Clin Oncol 2015; 33: 3130-3136.
- Chen HH, Chen WT, Lee HC, Lin JK, Fang CY, Chou YH, Lin PC, Lin BW, Huang CC, Yeh CH, Hsu HH, Chen HC, Ting WC, Yang MC, Tan EC. Health-related quality of life and cost comparison of adjuvant capecitabine versus 5-fluorouracil/leucovorin in stage III colorectal cancer patients. Qual Life Res 2015; 24: 473-484.
- Tang L, Shen J, Wu MH. Clinical observation for combining capecitabine and navelbine to treat recurrent and metastatic breast cancer. Chin ClinOncol 2010; 15: 67-69.
- Li Y, Martin RC 2nd. Herbal medicine and hepatocellular carcinoma: applications and challenges. Evid Based Complement Alternat Med 2011; 2011: 541209.
- Yin LR, Chen ZX, Zhang SJ, Sun BG, Liu YD, Huang HZ. Expression of phosphatase and tensin homolog deleted on chromosome ten in liver of athymic mice with hepatocellular carcinoma and the effect of Fuzheng Jiedu Decoction. World J Gastroenterol 2008; 14: 108-113.
- Yu ZM, Huang XH, Yan CQ, Gao J, Liang ZS. Effect of Fuzheng Jiedu granule on immunological function and level of immune-related cytokines in immune-suppressed mice. J Integr Arg 2016; 15: 650-657.
- Kurokawa Y, Sugimoto N, Miwa H, Tsuda M, Nishina S, Okuda H, Imamura H, Gamoh M, Sakai D, Shimokawa T, Komatsu Y, Doki Y, Tsujinaka T, Furukawa H. Phase II study of trastuzumab in combination with S-1 plus cisplatin in HER2-positive gastric cancer (HERBIS-1). Br J Cancer 2014; 110: 1163-1168.
- Lordick F, Lorenzen S, Yamada Y, Ilson D. Optimal chemotherapy for advanced gastric cancer: is there a global consensus? Gastric Cancer 2014; 17: 213-225.
- Li Z. Chemotherapy and immunotherapy in malignancies. Beijing: Peoples Health Publ H 1990; 58-65.
- Li LM. Curing 30 patients with metastatic breast cancer by using the combination of docetaxel and capecitabine. Cancer Res Clin 2011; 23: 628-629.
- Guimbaud R, Louvet C, Ries P, Ychou M, Maillard E, André T, Gornet JM, Aparicio T, Nguyen S, Azzedine A, Etienne PL, Boucher E, Rebischung C, Hammel P, Rougier P, Bedenne L, Bouché O. Prospective, randomized, multicenter, phase III study of fluorouracil, leucovorin, and irinotecan versus epirubicin, cisplatin, and capecitabine in advanced gastric adenocarcinoma: a French intergroup (Fédération Francophone de Cancérologie Digestive, FédérationNationale des Centres de LutteContre le Cancer, and GroupeCoopérateurMultidisciplinaire en Oncologie) study. J ClinOncol 2014; 32: 3520-3526.
- Okines AF, Langley RE, Thompson LC, Stenning SP, Stevenson L, Falk S, Seymour M, Coxon F, Middleton GW, Smith D, Evans L, Slater S, Waters J, Ford D, Hall M, Iveson TJ, Petty RD, Plummer C, Allum WH, Blazeby JM, Griffin M, Cunningham D. Bevacizumab with peri-operative epirubicin, cisplatin and capecitabine (ECX) in localised gastro-oesophageal adenocarcinoma: a safety report. Ann Oncol 2013; 24: 702-709.
- Azuma Y, Hata K, Sai K, Udagawa R, Hirakawa A, Tohkin M, Ryushima Y, Makino Y, Yokote N, Morikawa N, Fujiwara Y, Saito Y, Yamamoto H. Significant association between hand-foot syndrome and efficacy of capecitabine in patients with metastatic breast cancer. Biol Pharm Bull 2012; 35:717-724.
- Zhu B, Wu JR, Zhou XP. A retrospective comparison of trastuzumab plus cisplatin and trastuzumab plus capecitabine in elderly HER2-positive advanced gastric cancer patients. Medicine (Baltimore) 2015; 94: e1428.
- Passoni P, Reni M, Cattaneo GM, Slim N, Cereda S, Balzano G, Castoldi R, Longobardi B, Bettinardi V, Gianolli L, Gusmini S, Staudacher C, Calandrino R, Di Muzio N. Hypofractionated image-guided IMRT in advanced pancreatic cancer with simultaneous integrated boost to infiltrated vessels concomitant with capecitabine: A phase I study. Int J Radiat Oncol Biol Phys 2013; 87: 1000-1006.
- Perri F, Muto P, Argenone A, Ionna F, Longo F, Fulciniti F, Sandomenico F, Daponte A, Caponigro F. Induction chemotherapy with docetaxel, cisplatin and capecitabine, followed by combined cetuximab and radiotherapy in patients with locally advanced inoperable squamous cell carcinoma of the head and neck: A phase I-II study. Oncology 2013; 84: 251-254.
- Yan S, Jiang X, Yang J, Yan D, Wang YX. Radiotherapy for nasopharyngeal carcinoma and combined capecitabine and nimotuzumab treatment for lung metastases in a liver transplantation recipient: A case experience of sustained complete response. Cancer BiotherRadiopharm 2012; 27: 519-523.
- Wheeler HE, González-Neira A, Pita G, de la Torre-Montero JC, Alonso R, Lopez-Fernandez LA, Alba E, Martín M, Dolan ME. Identification of genetic variants associated with capecitabine-induced hand-foot syndrome through integration of patient and cell line genomic analyses. Pharmacogenet Genomics 2014; 24: 231-237.
- Pierga JY, Bidard FC, Cropet C, Tresca P, Dalenc F, Romieu G, Campone M, Mahier Ait-Oukhatar C, Le Rhun E, Gonçalves A, Leheurteur M, Dômont J, Gutierrez M, Curé H, Ferrero JM, Labbe-Devilliers C, Bachelot T. Circulating tumor cells and brain metastasis outcome in patients with HER2-positive breast cancer: The LANDSCAPE trial. Ann Oncol 2013; 24: 2999-3004.
- Jin K, Lan H, Xie B, He K, Xu Z, Li G, Han N, Teng L, Cao F. Antitumor effects of FP3 in combination with capecitabine on PDTT xenograft models of primary colon carcinoma and related lymphatic and hepatic metastases. Cancer Biol Ther 2012; 13: 737-744.