Research Article - Biomedical Research (2017) Volume 28, Issue 11
The value of contrast-enhanced ultrasound in evaluating the effect of uterine artery embolisation in morbidly adherent placenta after delivery
Guanchun Chen1,2, Junhu Chen3, Yanyan Wang4, Suijin Zheng4, Hongmei Liu3, Guozhen Liu2, Huaiyuan Zhou2 and Mei Zhong1*
1Department of Obstetrics and Gynecology, Nanfang Hospital, Southern Medical University, Guangzhou, PR China
2Department of Medical Ultrasonics, Affiliated HouJie Hospital of Guang Dong Medical University, Dongguan, PR China
3Guangdong Provincial Institute of Biological Products and Materia, Guangzhou, PR China
4Department of Obstetrics and Gynecology, Affiliated HouJie Hospital of Guang Dong Medical University, Dongguan, PR China
- *Corresponding Author:
- Mei Zhong
Department of Obstetrics and Gynecology
Nanfang Hospital
Southern Medical University, PR China
Accepted on March 22, 2017
Abstract
The purpose of this study was to explore the clinical value of Contrast-Enhanced Ultrasound (CEUS) using a microbubble contrast agent in evaluating placental vascularity and the effect of Uterine Artery Embolisation (UAE) treatment in morbidly adherent placenta after delivery. Twenty two cases of morbidly adherent placenta accreta after delivery were examined by gray-scale ultrasound, Color Doppler imaging, and CEUS. All the patients undergone UAE combining with Methotrexate (MTX) and were followed up by CEUS. The images of the CEUS before and after UAE were compared. The time that the enhancement began before UAE in the abnormal mass was earlier than that in the myometrium (abnormal mass 10.4 ± 1.7 s, myometrium 12.0 ± 2.1 s; p=0.007). The duration of enhancement before UAE in the abnormal mass was longer than that in the myometrium (262.0 ± 18.3 s vs. 174.0 ± 15.9 s; p=0.000). The PI and the abnormal mass before UAE were higher than that of the normal myometrium (25.8 dB ± 3.2 dB vs. 14.0 dB ± 2.8 dB; P=0.000). The abnormal mass displayed hyperenhancement before UAE. The abnormal mass displayed non-enhancement in 18 cases and isoenhancement in 4 cases after UAE. The 18 cases of nonenhancement expelled the placenta by Dilation and Curettage (D and C) completely 7 days after UAE and the 4 cases of isoenhancement expelled part of the placenta by D and C and hysteroscope were performed 30 days after UAE. CEUS may be a useful tool for the quantitative assessment of uteroplacental vascularity in morbidly adherent placenta after delivery and may be used to follow up the conservative treatment.
Keywords
Contrast-enhanced ultrasound, Quantitative analysis, Time-intensity curve, Morbidly adherent placenta, Uteroplacental vascularity, Uterine artery embolisation
Introduction
Morbidly adherent placenta is a potentially life-threatening obstetrical emergency. It occurs when there is abnormal adherence of the placenta to the uterine wall due to a defect in the decidua basalis [1,2]. The incidence of morbidly adherent placenta has risen 13-fold since the early 1900s in parallel with rising Caesarean section rates [3]. Morbidly adherent placenta may cause severe maternal morbidity, including conditions such as uterine perforation, infection, and severe hemorrhage. Severe bleeding is the single most significant factor of maternal death worldwide.
Although abdominal hysterecyomy is the most definitive management regularly, conservative management not only can preserve the patients’ fertility, but also can reduce morbidity and lower incidence of hysterectomy, blood transfusion, disseminated intravascular coagulation and severe postpartum haemorrhage. Uterine artery embolisation may reduce the incidence of massive haemorrhage causing by abnormal and dilated vessels underlying the basal plate. It is important to evaluate the vascularity of morbidly adherent placenta during the conservative treatment.
Grey scale and color Doppler imaging are commonly used to monitor the treatment effect of morbidly adherent placenta. But it is difficult to evaluate placental invasion into the myometrium by grey-scale sonography. Color or Power Doppler imaging is another modality that may be used to visualize and evaluate vascular extension from the placental bed. However, this modality produces faint signals when blood flows at a low velocity, which may inhibit accurate evaluation of uteroplacental vascularity of morbidly adherent placenta. It is sometimes difficult to differentiate placenta retention such as necrotic decidua and blood clots from morbidly adherent placenta by grey-scale and color Doppler ultrasound. Magnetic Resonance Imaging (MRI) has also been proposed as a diagnostic tool for morbidly adherent placenta, but it is a more expensive procedure and inconvenient than ultrasonography.
CEUS using a microbubble contrast material is a technique that overcomes some of the limitations of Doppler ultrasound. CEUS has been used to assess microvascular flow pattern in tumorous lesions in the kidney, and in the cerebrum [4-6]. Quantitative CEUS curves can be characterized by parameters such as time to peak and maximum intensity. Although these parameters are indirect measures of perfusion, they facilitate reproducible and clinically-feasible estimation of tissue perfusion [7]. The purpose of this study was to employ CEUS for the quantitative assessment of uteroplacental vascularity in morbidly adherent placenta after delivery and follow up the conservative treatment to reduce complications and preserve the patient’s fertility.
Methods
Between July 2012 and July 2016, 22 cases of morbidly placenta acceta were examined with Gray-scale ultrasound, color Doppler imaging and CEUS. Microvascular perfusion was evaluated, and enhanced features of abnormal mass, myometrium, and the serous layer were observed. The mean age of the patients was 27.5 ± 5.2 years (range 20-43 years). The mean gestational week was 22.2 ± 9.2 weeks (range 12 and 40 weeks). Four patients had histories of caesarean section, and 18 patients had histories of induced abortion, from one to five times.
We defined morbidly adherent placenta by the presence of one or more of the following clinical: (a) Difficult, manual, piecemeal removal of the placenta, performed if there was no evidence of placental separation at least 20 min after parturition despite active management of the third stage of labor; (b)The symptoms such as irregular vaginal bleeding and abnormal menstruation; the repeated sonographic evidence of persistent products of conception having images consistent with placenta accrete [8]. (c) The MRI evidence of morbidly adherent placenta (d) histological observations.
Materials and methods
All patients were examined with a Philips iU22 ultrasound system (Philips Healthcare, Andover, MA, USA).
Gray scale and color Doppler ultrasonography (US) examination
Using two-dimensional (2D) grey scale/color Doppler transabdominal and transvaginal US, we investigated the following: (I) the size of the uterus, (II) the location, size, boundary, and color Doppler imaging of the intrauterine abnormal mass; and (III) the relationship between the abnormal mass and anterior wall of the uterus, and the relationship between the abnormal mass and the urinary bladder. Transabdominal US was performed with a 1.0-5.0 MHz (C5-1) wideband convex transducer (Philips Healthcare, Andover, MA, USA) and transvaginal US was performed with a 3D9-3V transducer (Philips Healthcare, Andover, MA, USA).
CEUS examination
CEUS was performed more than 20 min after delivery using a Philips iU22 ultrasound system (Philips Healthcare, Andover, MA, USA). A 1.0-5.0 MHz (C5-1) wideband convex transducer (Philips Healthcare, Andover, MA, USA) was used, applying the following settings in all cases: mean depth, 16 cm; mean angle of view, 60; and mechanical index, 0.08.
A 1.0 ml bolus of contrast agent (Sono Vue, Bracco Imaging SpA, Milan, Italy) was administered intravenously, followed by flushing with 5 mL of saline. During the examination, the transducer was held in a fixed position for three minutes. All data from examinations were digitally stored. The investigation continued during the whole enhancement process. The time that the enhancement began, the duration of enhancement were recorded.
Data processing
Time-acoustic intensity curves were analysed automatically by the quantitative analysis software package QLAB (Philips Healthcare, Andover, MA, USA). A manually designed Region of Interest (ROI) was placed around the enhanced area of the abnormal mass and over the normal myometrium. Gamma variate fitting (a statistical model used to normalize the dispersion of gamma values in a perfusion study) of the intensity curves from the myometrium and abnormal mass was performed. The Peak Intensity (PI) defined as the highest value of the time-intensity curve [9], were calculated automatically.
UAE treatment
Pelvic embolisation was performed by a well-trained staffradiologist. Catheterisation of internal iliac arteries was made using a 5-F RUCcatheter (Beacon®Tip Torcon NB Radifocus; Cook Corporation, USA). Bilateral internal iliac arteryangiography was performed to study the bleeding uterus. Selective catheterisation of uterine artery was attempted. 100-200 mg MTX was injected into the local bleeding utery and then Gelatine sponge pledgets with the diameter measuring from 1 mm to 3 mm were used to occlude the bleeding uterine artery.
All the 22 cases were followed up both by CEUS and conventional (grey and color Doppler) ultrasound after conservative treatment.
The study was approved by the institutional review board and ethics committee of Affiliated HouJie Hospital of Guang Dong Medical University, and all patients provided their written informed consent for our management protocols.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software (IBM Corp., Armonk, NY, USA). The time that the enhancement began, the duration of enhancement, PI of the abnormal mass and the normal myometrium were analysed with the T-test in the 22 patients. Values with P<0.05 were considered to be statistically significant. Data were expressed as mean ± standard deviation.
Results
CEUS images and conventional ultrasound images before UAE
Features observed by conventional ultrasound: Grey-scale ultrasound showed abnormal masses in the uterus with similar echogenicity or hyperechogenicity as that of myometrium. The adjacent myometrium was thinner than normal. The mass exhibited no capsule, and there was no distinct boundary between the mass and the myometrium. Blood flow was detected both around and in the abnormal mass in 18 cases. No blood flow signal was detected around and in the abnormal mass in 4 cases.
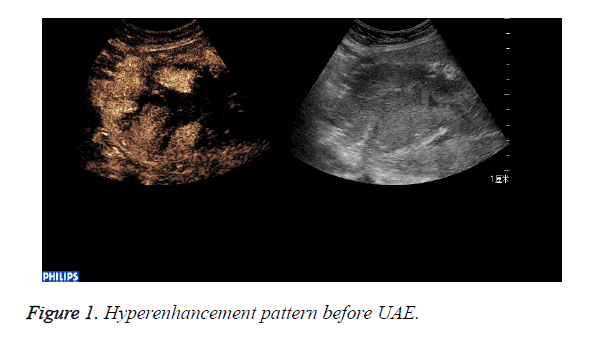
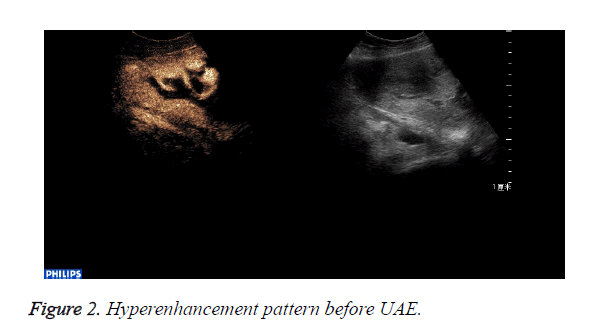
CEUS perfusion imaging (Figures 1 and 2): All the 22 patients enhanced both in the abnormal mass and the myometrium. The serous layer, the abnormal mass and their adjacent myometrium, and the normal myometrium enhanced in turn. There was no obvious demarcation between the abnormal mass and adjacent myometrium. The abnormal mass and adjacent myometrium enhanced later than the serous layer but slightly earlier than the normal myometrium. The time that the enhancement began was (abnormal mass 10.4 ± 1.7 s, myometrium 12.0 ± 2.1 s; p=0.007). The abnormal mass showed hyperenhancement by visual investigation. The duration of abnormal mass enhancement was longer than that of the normal myometrium. The duration of enhancement was (abnormal mass 262.0 ± 18.3 s, myometrium 174.0 ± 15.9 s; p=0.000), and the abnormal mass degraded later than the myometrium. The enhancement of the abnormal mass showed quick wash-in and slow wash-out. Some sections inside the mass displayed non-enhancement during the examination (Table 1).
| Location | PI (dB) | Duration of enhancement(s) | Time that the enhancement began(s) |
|---|---|---|---|
| Lesions | 25.8 ± 3.2 | 262.0 ± 18.3 | 10.4 ± 1.7 |
| Normal myometrium | 14.0 ± 2.8 | 174.0 ± 15.9 | 12.0 ± 2.1 |
| t/z | 12.8 | 17.8 | 2.8 |
| p | 0 | 0 | 0.007 |
Table 1. Comparison of parameters between the lesions and the normal myometrium before UAE.
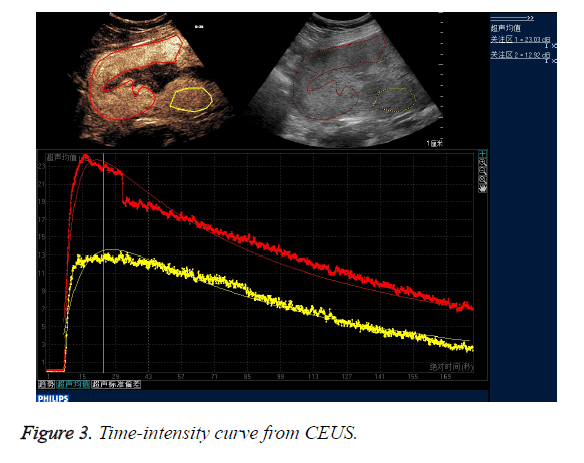
CEUS time-acoustic intensity curves before treatment were obtained for all patients: The time-acoustic intensity curve showed a rapidly-rising slope from the time of the bolus injection to the time of PI, and a gradual descending slope after reaching PI in the abnormal mass (Figure 3). The PI values of the abnormal mass were significantly higher than those of the normal myometrium (25.8 dB ± 3.2 dB vs. 14.0 dB ± 2.8 dB; P=0.000).
CEUS images and conventional ultrasound images were followed up 7 days after UAE treatment
Grey-scale images after treatment were similar to these before treatment: No blood flow was detected around and in the abnormal mass in 21 cases and blood flow was detected around the abnormal mass in one case.
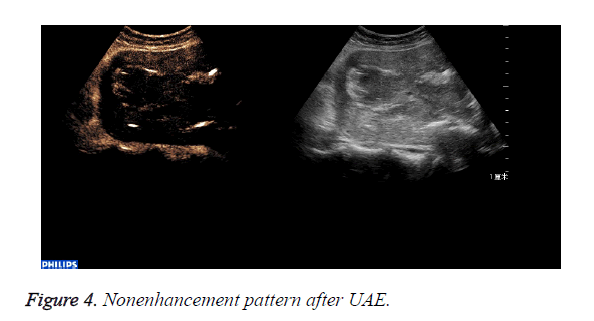
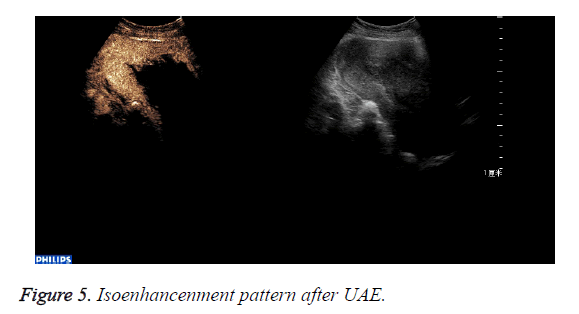
The abnormal mass displayed non-enhancement in 18 cases (Figure 4): The 18 cases expelled the placenta by D andC completedly 7days after UAE. Part of the abnormal mass displayed isoenhancement in 4 cases (Figure 5). The serous layer, the normal myometrium, the abnormal mass enhanced in turn. The range of the enhancement was smaller than that before the treatment. The 4 cases eradicated part of the placenta by Dand C and hysteroscope were performed 30 days after UAE. All the patients recovered to normal condition within two months.
Discussion
The most important complication of abnormally invasive placentation is massive hemorrhage resulting from attempted manual placental separation from its poorly formed decidual bed, where the abnormal large-caliber spiral vessels opens up. Conservative management of invasive placenta may result in a lower incidence of hysterectomy. Conceptually, conservative management aimed at avoiding uterine bleeding by leaving in place the abnormal and dilated vascular channels underlying the basal plate. However, postpartum haemorrhage may still occur so that UAE may be needed [10-12].
UAE treatment has the advantages of avoiding immediate massive haemorrhage, avoiding damage to surrounding structures and the potential preservation of a functional uterus which may allow subsequent successful pregnancies UAE has a well-established efficacy for the treatment of postpartum hemorrhage in women with placenta accreta or percreta [13-15]. A 2011 review article which reported 45 patients managed with UAE estimated that a subsequent menstruation in 8/13 (62%) and a subsequent pregnancy in 5/33 (15%), and All patients survived until the end of follow up [16].
Morbidly adherent placenta is a pregnancy complication characterized by the presence of life-threatening uteroplacental neovascularization. One possible role for UAE is to decrease the vascularity of the remaining placenta or placental portions and consequently make secondary hysterectomy easier [11].
UAE Nevertheless, severe postpartum haemorrhage related to invasive placenta remains challenging [10,17].
Evaluation of uteroplacental neovascularization in morbidly adherent placenta may help to monitor the UAE treatment effect to reduce complications.
The RCOG guidelines specify ultrasonography as the preferred imaging modality for follow-up, to monitor the degree and vascularity of the placental attachment to the uterus [18]. But sometimes it is difficult to differentiate between morbidly adherent placenta and necrotic tissue as well as blood clots and it is difficult to judge whether the placenta separate from the uterus wall and to assess the range of the placenta. CEUS is recognized as a valuable method for assessing neovasculature changes due to the characteristics of the US contrast agents, which are pure blood tracers. The US contrast agents travel at the same velocity as blood and can be relatively stable over a period of time suitable for ultrasonographic examination [19]. All the 22 patients enhanced both in the abnormal mass and the myometrium by CEUS. While no blood flow signal was detected around and in the abnormal mass in 4 cases by color Doppler imaging before treatment. CEUS reflects perfusion of the placenta more accurately than color Doppler imaging.
Moreover, this method is inexpensive, non-invasive, and easy to use even at the bedside; it does not involve ionizing radiation; and it can be conveniently performed several times in a short period.
Time-acoustic Intensity Curve (TIC) reflects the dynamic variation of the concentration of the ultrasound contrast agents in the microcirculation. TIC parameters are descriptors for blood volume perfusion and have been experimentally shown to reflect microvasculature flow [20]. The PI is a measure of the relative blood volume in the scanned tissue. The fact that the PI of the abnormal mass was significant higher than that of the normal myometrium may be explained by the invasion of extravillous cytotrophoblasts into large arteries of the uterus. US contrast agents may rapidly accumulate in the intervillous space due to pulsatile, high-pressure blood flow. Higher PI values correspond to higher concentrations of contrast agents. In this study, the PI of the abnormal mass was higher than that of the normal myometrium, reflecting higher perfusion and a higher concentration of contrast agents in the placental bed compared to the normal myometrium. Due to the abnormal uteroplacental vasculature and the distribution kinetics of the contrast agents in the placenta, the enhanced patterns of the CEUS perfusion imaging showed quick wash-in and slow wash-out and hyperenhancement.
Methotrexate has played an efficient role in the conservative management of ectopic pregnancy, cornual pregnancy, and morbidly adherent placenta [21]. Methotrexate is an antimetabolite that interferes with the synthesis of DNA and interrupts the synthesis of the purine nucleotide thymidilate and the amino acids serine and methionine. MTX is assumed to affect the placental tissue by reducing its vascularity.
UAE allowed the control of uterine bleeding accompanied with a gradual decrease in vascularity at the site of increta [22]. UAE combining with MTX local injection is effective at reducing the uteroplacental blood flow to further induce thrombosis of the intervillous space and to achieve necrosis of retained placental tissue.
Our results confirm that UAE results in an overall decreased placental vascularity as evidenced on the CEUS imaging after treatment. The abnormal mass displayed non-enhancement in 18 cases and displayed isoenhancement in 4 cases, however the level of enhancement in the abnormal mass were lower than before treatment. No blood flow was detected around and in the abnormal mass in 21 cases and blood flow was detected around the abnormal mass in one case by color Doppler imaging.
After UAE the perfusion of the uterus and placenta was blocked completely, so that the abnormal mass displayed non-enhancement in 18 cases and the placentas were expulsed completely by D and C.
The iso-enhancement pattern in the four cases may be due to rich collateral feeding vessels arising from cervicovaginal branches of the uterine ateries. Moreover insufficient blockage of uteroplacental circulation may occur during UAE because low intervillous resistance helps maintain persistent uteroplacental blood flow via physiological anastomoses and newly developed arteries [10].
The study by Krapp et al. [23] demonstrates that decrease and final cessation of blood flow between the uterine wall and the placenta marks the end of the latent phase of placental separation. Persistent blood flow due to abnormal vascular connections indicates abnormal placentation, and specifically placenta accreta. The changes of the enhancement pattern reflect the treatment effect. The iso-enhancement pattern of the abnormal mass after UAE indicates a persisting uteroplacental circulation, which suggests that neovascularization still exists and that the uterus need to be followed up again and conservative treatment should to be continued to prevent from secondary bleeding, although blood flow was detected around abnormal mass only in one case by color Doppler imaging. CEUS can provide more accurate quantitative information than grey scale and color Doppler imaging in the evaluation of the UAE treatment effect of morbidly adherent placenta.
In conclusion, CEUS provides information on the perfusion features of the pathological placenta, which may help to determine the position of the placenta and differentiate between nonnecrotic placenta and necrotic placenta tissue or hemotocele, and to monitor the change of the perfusion of the placenta. CEUS may therefore be useful for the assessment of uteroplacental vascularity in morbidly adherent placenta for conservative therapy. The potential role Of CEUS would be to monitor the decrease of placental vascularity. Consequently, CEUS would help distinguish between women at risk for secondary bleeding and those for whom his risk is negligible. Moreover, CEUS allows objective evaluation of the degree of devascularization of retained placental tissue.
Conflicts of Interest of Statement
None declared.
Acknowledgements
We would like to express our gratitude to all the subjects who participated in this current study.
References
- Mazouni C, Gorincour G, Juhan V, Bretelle F. Placenta accreta: a review of current advances in prenatal diagnosis. Placenta 2007; 28: 599-603.
- Stirnemann JJ, Mousty E, Chalouhi G, Salomon LJ, Bernard JP and Ville Y. Screening for placenta accreta at 11-14 weeks of gestation. Am J obstetr Gynecol 2011; 205: 541-546.
- Wortman AC, Alexander JM. Placenta accreta, increta, and percreta. Obstet Gynecol Clin North Am 2013; 40: 137-154.
- Nemec U, Nemec SF, Novotny C, Weber M, Czerny C, Krestan CR. Quantitative evaluation of contrast-enhanced ultrasound after intravenous administration of a microbubble contrast agent for differentiation of benign and malignant thyroid nodules: assessment of diagnostic accuracy. Eur Radiol 2012; 22: 1357-1365.
- Schneider AG, Hofmann L, Wuerzner G, Glatz N, Maillard M, Meuwly JY, Eggimann P, Burnier M, Vogt B. Renal perfusion evaluation with contrast-enhanced ultrasonography. Nephrol Dial Publ Eur Dial Transpl Eur Renal Assoc 2012; 27: 674-681.
- van Wijk MC, Klaessens JH, Hopman JC, Liem KD, Thijssen JM. Assessment of local changes of cerebral perfusion and blood concentration by ultrasound harmonic B-mode contrast measurement in piglet. Ultrasound Med Biol 2003; 29: 1253-1260.
- Delorme S, Krix M. Contrast-enhanced ultrasound for examining tumor biology. Cancer Off Publ Int Canc Imag Soc 2006; 6: 148-152.
- Palacios-Jaraquemada JM. Diagnosis and management of placenta accreta. Best Pract Res Clin Obstet Gynaecol 2008; 22: 1133-1148.
- Ignee A, Jedrejczyk M, Schuessler G, Jakubowski W, Dietrich CF. Quantitative contrast enhanced ultrasound of the liver for time intensity curves-reliability and potential sources of errors. Eur J Radiol 2010; 73: 153-158.
- Soyer P, Morel O, Fargeaudou Y, Sirol M, Staub F. Value of pelvic embolization in the management of severe postpartum hemorrhage due to placenta accreta, increta or percreta. Eur J Radiol 2011; 80: 729-735.
- Teo SB, Kanagalingam D, Tan HK, Tan LK. Massive postpartum haemorrhage after uterus-conserving surgery in placenta percreta: the danger of the partial placenta percreta. BJOG 2008; 115: 789-792.
- La Folie T, Vidal V, Mehanna M, Capelle M, Jaquier A. Results of endovascular treatment in cases of abnormal placentation with post-partum hemorrhage. J Obstet Gynaecol Res 2007; 33: 624-630.
- Diop AN, Bros S, Chabrot P, Gallot D, Boyer L. Placenta percreta: urologic complication after successful conservative management by uterine arterial embolization: a case report. Am J Obstet Gynecol 2009; 201: e7-8.
- Timmermans S, van Hof AC, Duvekot JJ. Conservative management of abnormally invasive placentation. Obstet Gynecol Surv 2007; 62: 529-539.
- Alanis M, Hurst BS, Marshburn PB, Matthews ML. Conservative management of placenta increta with selective arterial embolization preserves future fertility and results in a favorable outcome in subsequent pregnancies. Fertil Steril 2006; 86: e3-7.
- Steins Bisschop CN, Schaap TP, Vogelvang TE, Scholten PC. Invasive placentation and uterus preserving treatment modalities: a systematic review. Arch Gynecol Obstet 2011; 284: 491-502.
- Bretelle F, Courbiere B, Mazouni C, Agostini A, Cravello L. Management of placenta accreta: morbidity and outcome. Eur J Obstet Gynecol Reprod Biol 2007; 133: 34-39.
- Royal College of Obstetricians and Gynaecologists. Green Top Guideline 27: Placenta praevia, placenta praevia accreta and vasa praevia: diagnosis and management. London RCOG 2011.
- Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 1998; 97: 473-483.
- Deng CX, Lizzi FL. A review of physical phenomena associated with ultrasonic contrast agents and illustrative clinical applications. Ultrasound Med Biol 2002; 28: 277-286.
- Wong HS, Parker S. Ultrasonographic findings in conservatively treated placenta percreta. Ultrasound Obstet Gynecol 2005; 26: 580-581.
- Philippe S, Marc S, Yann F, Laurence B, Olivier M, Anthony D, Mourad B, Etienne G, Delphine H, Emmanuel B, Olivier D. Placental vascularity and resorption delay after conservative management of invasive placenta: MR imaging evaluation. Eur Soc Radiol 2013; 23: 262-271.
- Krapp M, Baschat AA, Hankeln M, Gembruch U. Gray scale and color Doppler sonography in the third stage of labor for early detection of failed placental separation. Ultrasound Obstet Gynecol 2000; 15: 138-142.