Case Report - Case Reports in Surgery and Invasive Procedures (2022) Volume 6, Issue 4
The transformed chymify-carcinoma pancreas.
Anubha Bajaj*Department of Consultant Histopathologist, A.B. Diagnostics, A-1, Ring Road, Rajouri Garden, New Delhi 110027, India
- *Corresponding Author:
- Anubha Bajaj
Department of Consultant Histopathologist
A.B. Diagnostics, A-1, Ring Road
Rajouri Garden, New Delhi 110027, India
E-mail: anubha.bajaj@gmail.com
Received: 13-May-2022, Manuscript No. AACRSIP-22-63768; Editor assigned: 16-May-2022, PreQC No. AACRSIP-22-63768(PQ); Reviewed: 30-May-2022, QC No. AACRSIP-22-63768; Revised: 04-June-2022, Manuscript No. AACRSIP-22-63768(R); Published: 10-June-2022, DOI: 10.35841/aacrsip-6.4.116
Citation: Bajaj A. The transformed chymify-carcinoma pancreas. Case Rep Surg Invasive Proced. 2022;6(4):116
Abstract
Carcinoma pancreas emerges from diverse pancreatic cells wherein pancreatic adenocarcinoma is preponderantly (~90%) discerned with mutations within KRAS, CDKN2A, TP53 AND SMAD4 genes. Smoking, obesity, type 2 diabetes mellitus, type 3c or pancreatogenic diabetes, excessive alcohol intake, chronic pancreatitis, hereditary pancreatitis, family history of carcinoma pancreas, consumption of processed or red meat and certain genetic profiles predispose to occurrence of carcinoma pancreas. Carcinoma pancreas manifests with anorexia, unexplained weight loss, indigestion, upper abdominal or back pain, jaundice, compression of adjacent organs, altered gastric emptying, nausea, vomiting, steatorrhoea, constipation, new onset type 2 diabetes mellitus, palpable abdominal mass or preceding pancreatitis.
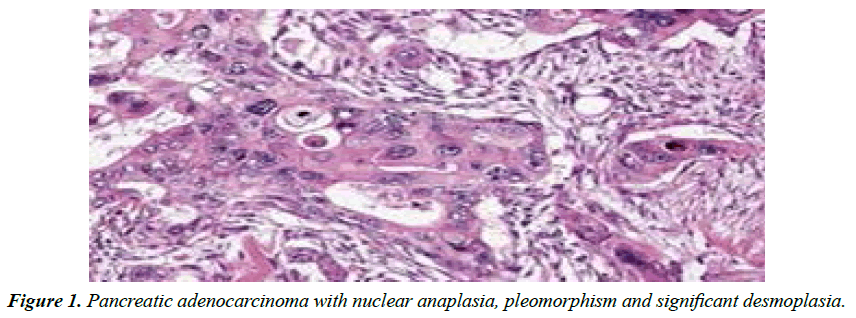
Adenocarcinoma pancreas demonstrates moderate to poorly differentiated glandular articulations with significant desmoplasia or dense, fibrotic stroma. Computerized Tomography (CT) or endoscopic ultrasound (EUS) can be employed to confirm carcinoma pancreas. Surgical resection can appropriately alleviate pancreatic adenocarcinoma although prognostic outcomes are inferior.
Keywords
Carcinoma pancreas, chymify, Adenocarcinoma pancres.
Introduction
Carcinoma pancreas emerges from diverse pancreatic cells and exemplifies possible regional and distant tumour infiltration. Pancreatic adenocarcinoma is preponderantly (~90%) discerned and arises from enzyme generating exocrine pancreas. Infiltrating pancreatic ductal adenocarcinoma commences within pancreatic ducts, frequently within head of pancreas [1,2].
Carcinoma pancreas emerging from exocrine region is preceded by several precancerous lesions such as pancreatic intraepithelial neoplasia, Intra-ductal Papillary Mucinous Neoplasm (IPMN), pancreatic Mucinous Cystic Neoplasm (MCN) or intra-ductal tubulopapillary neoplasm. Pancreatic adenocarcinoma enunciates mutations within KRAS, CDKN2A, TP53 AND SMAD4 genes. Besides mutation or deletion within SWI/SNF genes is exemplified. Hormoneproducing cells of endocrine pancreas engender minimally aggressive pancreatic Neuroendocrine Tumours (NET). Pancreatic neuroendocrine tumour occurs as a ‘functioning’ or ‘non-functioning’ neoplasm, contingent to hormone production [1,2].
Functioning neoplasms secrete hormones such as insulin, gastrin or glucagon, denominated as insulinoma, gastrinoma or glucagonoma, respectively. Clinical symptoms pertain to category of secreted hormone. Pancreatic neuroendocrine tumour exhibits genomic mutation of MEN1, DAXX, mTOR and ATRX genes. Pancreatic acinar cell carcinoma contributes to ~5% of exocrine neoplasms and exhibits enzyme producing, cellular clusters. Cystadenocarcinoma manifests ~1% of exocrine pancreatic neoplasms and demonstrates a superior prognosis. Exceptional pancreatoblastoma is commonly discerned in children and delineates a favourable outcome [1,2].
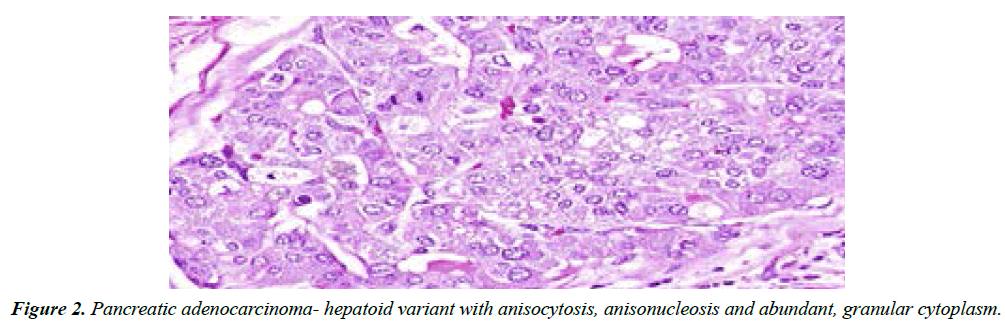
Additionally, adenosquamous carcinoma, signet ring carcinoma, hepatoid carcinoma, colloid carcinoma, undifferentiated carcinoma; undifferentiated carcinoma with osteoclast-like giant cells, solid pseudo-papillary tumour, pancreatic serous cystadenoma or pancreatic mucinous cystic neoplasm may emerge. Preliminary tumour discernment exhibits proportionate 5 year survival of around ~20% whereas neuroendocrine neoplasms demonstrate a proportion of ~65%.
Factors such as smoking, obesity, type 2 diabetes mellitus, type 3c or pancreatogenic diabetes, excessive alcohol intake with chronic pancreatitis, hereditary pancreatitis, an inherited component with family history of carcinoma pancreas, consumption of processed or red meat and certain genetic profiles contribute to occurrence of carcinoma pancreas. Carcinoma pancreas is associated with exceptional, hereditary syndromes such as Peutz-Jeghers syndrome, dysplastic nevus syndrome or familial atypical multiple mole and melanoma syndrome, ataxia-telangiectasia, autosomal dominant mutations of BRCA2 and PALB2 genes, hereditary non polyposis colon cancer or Lynch syndrome and familial adenomatous polyposis. Pancreatic neuroendocrine tumour is associated with Multiple Endocrine Neoplasia (MEN1) and von Hippel Lindau syndrome. Carcinoma pancreas usually occurs beyond 40 years and elderly individuals are commonly incriminated. A mild male predominance is observed Clinical symptoms pertain to initial tumour location within head of pancreas, neck, tapering body or tail Carcinoma pancreas manifests with anorexia, unexplained weight loss, inadequate digestion, decimated exocrine activity, cutaneous discoloration, upper abdominal pain radiating to back, jaundice, compression of adjacent organs, altered gastric emptying, nausea, bloating, vomiting, steatorrhoea, constipation, new onset type 2 diabetes mellitus, depression, weakness, dry mouth, insomnia, palpable abdominal mass or preceding pancreatitis. Clinical symptoms appear with tumour expansion beyond the pancreas. Antecedent tumefaction appears asymptomatic ‘Trousseau’s syndrome’ with portal vein thrombosis, superficial or deep vein thrombosis or thrombophlebitis may accompany carcinoma pancreas. ‘Courvoisier’s sign’ with jaundice accompanied by painless, oedematous gall bladder may indicate carcinoma pancreas. Typically, adenocarcinoma pancreas demonstrates moderate to poorly differentiated glandular articulations with significant desmoplasia, dense, fibrotic stroma incorporating cellular subtypes as myofibroblasts, macrophages, lymphocytes and mast cells admixed with type I collagen and hyaluronic acid. Pancreatic acinar cell carcinoma exhibits a granular appearance. Adeno-squamous pancreatic carcinoma exemplifies amalgamation of glands and squamous epithelial aggregates [1,2].
Pertinent tumour staging is obtained with TNM classification (Table 1). Pancreatic neuroendocrine tumour displays nests of tumour cells and is categorized contingent to cellular differentiation as well differentiated ‘G1’ to poorly differentiated ‘G3’ neoplasms.
| Tumour (T) | Node(N) | Metastasis(M) |
|---|---|---|
| T(is): Carcinoma in situ | N0: Regional lymph nodes lack carcinoma cells | M0:no distant metastasis |
| T1: Carcinoma confined to pancreas • T1a: Carcinoma <0.5cm • T1b:Carcinoma between0.5cm to 1 cm • T1c:Carcinoma between 1cm to 2 cm |
N1: Regional lymph node(~3) imbued with carcinoma cells | M1: Metastasis to distant organs as liver |
| T2:Carcinoma between 2cm to 4cm | ||
| T3:Carcinoma exceeding 4 cm, confined to pancreas | ||
| T4:Carcinoma extending beyond pancreas, into adjacent enlarged blood vessels |
Table 1. Tnm classification of carcinoma pancreas.
Computerized Tomography (CT) or Endoscopic Ultrasound (EUS) can be employed to confirm carcinoma pancreas and assess extent of tumour resection. Magnetic Resonance Imaging (MRI), Positron Emission Tomography (PET) or Magnetic Resonance Cholangiopancreatography (MRCP) may be adopted for tumour detection (Abdominal ultrasonography is beneficial in assessing hepatic metastasis and malignant ascites(Ultrasound guided fine needle aspiration can be employed for neoplastic cytological evaluation. Liver Function Tests (LFT) indicate conjugated hyper-bilirubinaemia and an elevated alkaline phosphatase or Ỹ-glutamyl transpeptidase. Tumour marker CA19-9 is frequently elevated in carcinoma pancreas. Typically, adenocarcinoma pancreas metastasizes to regional lymph nodes, hepatic parenchyma, peritoneal cavity, large intestine or pulmonary parenchyma. Tumour deposits within bone or brain are uncommon. Surgical resection can appropriately alleviate pancreatic adenocarcinoma (Figure 1), especially contemporary neoplasms. Precise tumour location and abutting vascular configurations require assessment ‘Whipple procedure’ comprised of pancreato-duodenectomy, gastro-jejunostomy and cholecysto-jejunostomy can be adopted for treating carcinoma head of pancreas [3,4].
Carcinoma tail of pancreas can be exterminated with distal pancreatectomy. Adjuvant chemotherapy or radiotherapy is beneficial in treating neoplasms amenable or unamenable to surgical resection. Chemotherapy with gemcitabine, 5 fluorouracil (5-FU) or erlotninb is adopted in borderline instances. Chemo-radiotherapy can be employed. Alternatively, FOLFIRINOX regimen or protein bound paclitaxel can be utilized. Miniature pancreatic neuroendocrine tumours below <1 centimetre magnitude can be managed with simple observation. Localized tumours confined to pancreas or with restricted metastasis are surgically eradicated [3,4].
Functioning tumours can be treated with octreotide or lanreotide. Targeted therapy with everolimus or sunitinib can decimate clinical symptoms and disease progression (Figure 2).
Radiotherapy can be adopted for tumour shrinkage or decimation of pain due to anatomic tumour extension as with bony metastasis. Besides, radiofrequency ablation, cryoablation or hepatic artery embolization can be employed. Liver transplantation may be contemplated in instances of liver metastasis [3,4].
Conclusion
Prognostic outcome of pancreatic adenocarcinoma and associated exocrine carcinomas is inferior. Pancreatic neuroendocrine tumour exhibits a rather favourable outcome. Enhanced expression of unfavourable genes as C-Met and MUC-1 enunciate an inferior outcome whereas expressivity of favourable genes as transcription factor PELP1 is associated with superior prognosis. Regular screening with endoscopic ultrasound, computerized tomography or magnetic resonance imaging is recommended in individuals with inherited or genetic neoplastic emergence
References
- Pijnappel EN, Dijksterhuis WPM. The fear of cancer recurrence and progression in patients with pancreatic cancer. Support Care Cancer. 2022;30(6):4879-87.
- Milella M, Bassi C. Evolving pancreatic cancer treatment: from diagnosis to healthcare management. Crit Rev Oncol Hematol. 2022;169:103571.
- Breunig M, Merkle J. Modelling plasticity and dysplasia of pancreatic ductal organoids derived from human pluripotent stem cells. Cell Stem Cell 2021;28:1105-24.
- Latenstein AEJ, van der Geest LGM. Nationwide trends in incidence, treatment and survival of pancreatic ductal adenocarcinoma. Eur J Cancer. 2020;125:83-93.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref