- Biomedical Research (2015) Volume 26, Issue 2
The toxic effect of nickel nanoparticles on oxidative stress and inflammatory markers.
Seyedeh tahereh razavipour1, Mohammadreza Behnammorshedi1, Razieh Razavipour2, Marziyeh Ajdary3*1Department of Biology, Payame Noor University, I.R. of IRAN
2International branch, Shiraz University of medical sciences, Shiraz, iran.
3Young Researchers & Elits Club, Khorasgan Branch, Islamic Azad University, Isfahan, Iran
- *Corresponding Author:
- Marziyeh Ajdary
3- Young Researchers & Elits Club
Khorasgan Branch
Islamic Azad University, Isfahan
Iran
Accepted date: February 17 2015
Abstract
Due to extensive application of nanoparticle in industries and medicine, their advantages and disadvantages have been paid attention to. In addition to useful applications, nanoparticles have different effects on body tissue. This study conducted to explore the toxic effect of nickel oxide nanoparticles on oxidative stress system and its damaging effect on the immune system of the rats. In this experimental study, 40 male Wistar rats were randomly divided into two groups. The experimental group received nickel oxide nanoparticles in the form of mixture with water in 25ppm dose for seven consecutive days. The control group received drinking water and food. After 7 days, the serum levels of INF-?, IL-4, MDA, CAT and GPX were measured and the data were analyzed using Independent t-test and SPSS-15 software. Interleukin-4 and interferon-? were significantly increased in the experimental group (25ppm) (P<0.05). MDA was also significantly increased in the experimental group compared with the control group (P<0.05). GPX and CAT were significantly reduced in the experimental group compared with the control group (P<0.05). Nanoparticles of nickel oxide resulted in the creation of free radicals and increased oxidative stress and inflammation in rats.
Keywords
Nickel oxide, cytokines, oxidative stress
Introduction
The application of nanoparticles in different branches of medicine and fundamental sciences has developed more, compared with the past. The size of these particles results in the formation of an increased surfactant, which in turn gives unique chemical, physical and biological characteristics to these particles. These particles do not face much problem when passing the biological barriers inside the body. Thus, they can be used as carriers for purposeful transportation of medicine and other materials into the target cells. Therefore, increased application of nanoparticles requires more study to explore its possible toxic effects on the target cells and adjacent cells. Donaldson et al. (2004) created a transformation in the field of toxicology by introducing the science of Nano toxicology [1]. Due to their extremely small size, nanoparticles could easily pass through the physiological barriers of the body and distributed the tissues through circulatory system [13]. Metal nanoparticles can cause the production of ROS. For example, the reaction O2 - +H2O2 (fe) → OH+OH-+O2 causes the production of hydroxyl radical, which is significantly active [2]. Production of ROS due to the presence of nanoparticles can cause serious and heritable damages to DNA. For example, chemical changes in histones or other proteins, which play a role in the formation of DNA, unwind the helical structure of DNA and exposed DNA to any change [3,4]. Mitochondrial genome is significantly vulnerable to oxidative attack [5]. Agarwal et al. (2003) suggested that high level of ROS results in breaking of the external and internal membranes of mitochondria ,whichconsequently results in cytochrome c being released from mitochondria. Activation of cascade of events stimulates apoptosis. ROS, therefore acts as a mediator [6]. Release of mitochondria proteins such as cytochrome c is controlled by protein members of bc12 family. Currently, 15 proteins of this family are identified in mammals and all of them have at least one of the four protected areas called bc12 homology domains. The members of this family can activate both anti-apoptotic and pro-apoptotic roles [7]. Mitochondrion is one of the main places for production of ROS in cell. Thus, its DNA is exposed to oxidative attack [8]. In a study conducted by Agarwal (2003), a negative relationship between ROS level and mitochondria membrane potential has been shown in a way that with the increase in ROS level, mitochondria membrane potential is decreased [6]. Immunotoxicity due to some toxins has a severe inhibitory effect on the activities of plasma’s cholinesterase and interleukin-2 [9]. Interleukin- 10 is an anti-inflammatory agent and is effective in reducing pathological signs resulted from toxins [10]. Thus, considering the lack of enough information regarding the toxic effects of nickel oxide on oxidative stress system, and some immune parameters, this study was designed to determine the toxic effect of nickel oxide nanoparticles on oxidative stress level and immunological parameters including determining the level of inflammatory and non-inflammatory cytokines in rats.
Materials and methods
Materials
The materials used in this study included NiO, which was purchased from Nuetrino Noavarane Nano Company. Ketamine, rat food, hematoxylin and laboratory kit were supplied by USCN Germany.
Devices
The devices used in this study included TEM (JEM- 200CX), ELISA Reader (HumaReader HS, Human company, Germany) and spectrophotometer (JENWAY, England).
Method
This study was conducted on forty 3-4 months old Wistar rats weighting about 200-250 g. The rats were purchased from the Center for Experimental Animals in Shahrekord and were randomly divided into two groups. Group A was defined as the experimental group and the other group was defined as controls. All the rats were kept in standard cages at 25 degrees Celsius and at 12-hours light/darkness cycle. Existing guidelines and the regulations passed by Moral Committee of Iran was followed. The experimental group received nickel oxide nanoparticles (Nuetrino Noavarane Nano company- Iran) for 7 consecutive days with 25 PPM concentration in the form of aqueous mixture. After 7 days of treatment, 3 – 4 ml blood samples were collected from the hearts under ketamine anesthesia from the hearts in test tubes without anticoagulant. The blood samples were centrifuged at 2000 rpm for 15 minutes. After separation of blood serum, the samples were distributed into micro tubes and kept at 70 degrees Celsius. The parameters such as catalase, glutathione peroxidase and malondialdehyde were measured by spectrophotometer. Interleukin-4 and interferon-Ɣ were measured using USCN laboratory kits by ELISA method.
The activity of enzyme CAT was measured using Aebi method [11]. Absolute ethanol (0.01 mml/mml) was added to a specific volume of tissue extract and incubated in ice for half an hour. Then Triton X-100 (10 percent) to make final concentration of one percent was added to it for measuring enzyme activity. The reaction was initiated by adding 30mM H2O2 to an appropriate volume of the tissue sample extract in 50 mM sodium phosphate buffer at PH=7. The absorption at 40 nm wavelength was recited and special activity was recited in unit of on milligram protein. The activity of glutathione peroxide of erythrocytes was measured indirectly through coupling reaction with glutathione reductase (GR). Reduction of oxidized glutathione resulted from glutathione peroxide reaction was estimated by consumption of NADPH and in the presence of GR. In this reaction, the oxidation of NADPH to NADP+ resulted in the reduction of absorption at 340nm wavelength, which is in proportion with glutathione peroxide (GPX) [12]. The level of malondialdehyde (MDA) in serum was measured using Satoh method. The reaction of malondialdehyde with thiobarbituric acid at boiling temperature resulted in pink color, which was extracted using n-butanol and its absorption was read at 530 nm wavelength. Standard curve of malondialdehyde was obtained using tetraethoxypropane 13]. For measuring cytokines and for conducting each test separately, micro tubes from freezer were removed till they melted completely at ambient temperature. Using ELISA, the samples and the standard were added to 96-well plate and were read at 450 nm wavelengths by ELISA Reader after completion of ELISA test steps. The reason for using 25PPM doses was for its less toxic effect compared with higher doses of 500, 250, 125 and 75 PPM doses, which could resulted in the death of rats in less than 24 hours. Therefore, 25PPM dose was chosen after testing different doses and it was found that nickel nanoparticles was highly toxic.
Preparation of nanoparticles: For preventing error, 25- PPM doses of nanoparticle suspension was prepared and mixed with distilled water.
Statistical analysis
Data were reported in the form of MEAN±SEM and were statistically analyzed using independent t-test and SPSS- 19 statistical software. The significance level of the tests was considered lower than 0.05.
Result
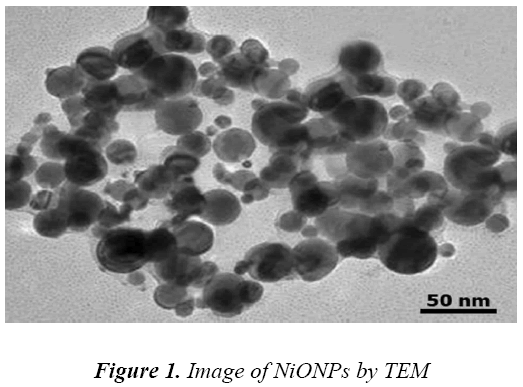
Microscopic characterization of NiO Nps
The morphology and size of the purchased NiONPs were controlled using transmission electron microscopyCM10 Philips (TEM). The images clearly shown that the average size of the particles was found to be in the order of 10 nm and they were relatively uniform in diameter with spherical shape (Fig 1). TEM consisted of a long column with source of electron rays on top that after transmitting.
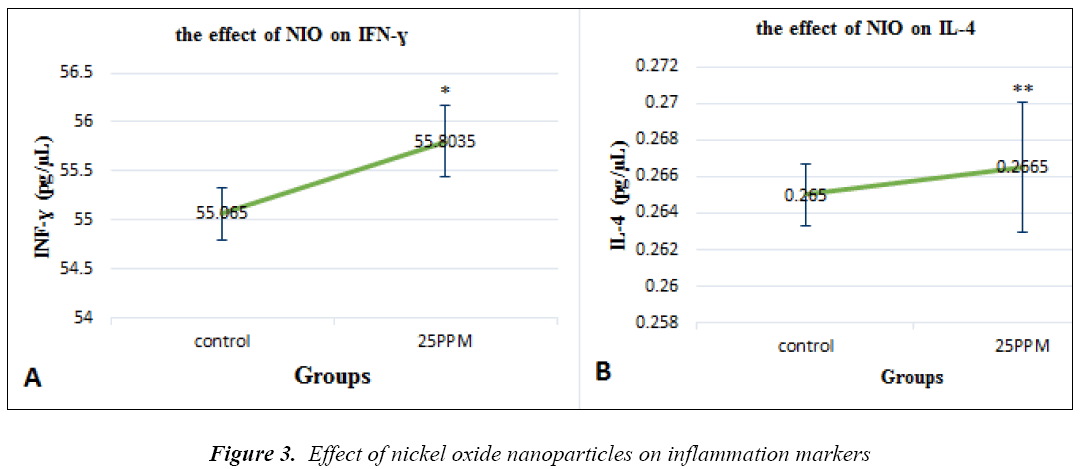
The toxic effects of nickel oxide nanoparticles on inflammatory factor
NiO nanoparticles are fond in the INF-ɣ factors (p=0.017). Its levels are show in groups receiving through the specimen, electron rays hit a photographic film or screen, built of fluorescent materials, and create an image. Since some rays do not pass through the sample, black spots were left on the image and, therefore electron microscope images looked black and white
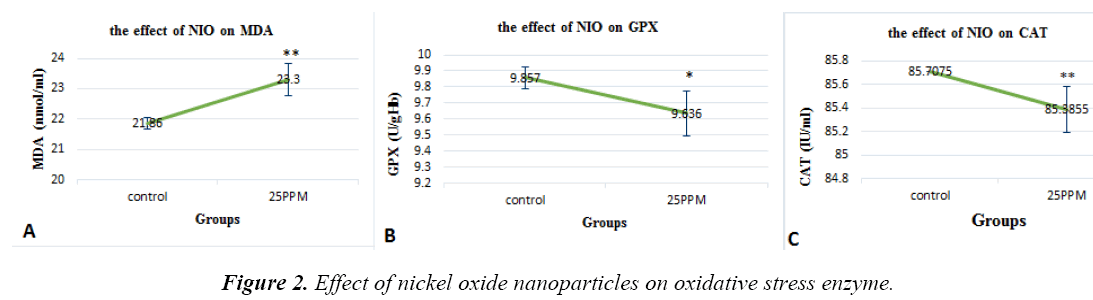
The toxic effects of nickel oxide nanoparticles on oxidative stress system
NiO nanoparticles are found in the MDA enzyme (p=0.002), and its levels are evident in groups receiving 25ppm of NiONP (Figure 2a). Glutathione peroxidase levels were significantly decreased compared with the controls (P = 0.012) (Figure 2B). Catalase levels were significantly decreased compared with the controls (P = 0.003) (Fig. 2C). 25ppm of NiONP (Figure 3a). IL-4 levels are shown in the groups that received nanoparticles of 25PPM, which were increased compared to controls (P = 0.007) (Fig. 3B).
Discussion
In the present study, the levels of glutathione peroxidase and catalase were decreased and malondialdehyde increased. Interleukin-4 and interferon-Ɣ were cytokines that were significantly increased in the experimental group, compared with the controls. Thus, increase of interleukin with an increase in nickel oxide nanoparticles could create disturbance in the production of interleukins by impacting the immune system in the body. A similar state of condition could also occur in the case of increased humoral immune response. Some toxins exerted impact on interleukin-4, 2., and others showed effects on interleukin 2 [14]. It should be noted that the decrease of these cytokines might be seen only in vitro and in the cells of a specific tissue [15]. Some nanoparticles, which were separated from different materials including different toxins, could result in change in antigen of body tissues in long term by being stored in those tissues [16]. It seems that in the in vivo state, the process might be decreased. We also noticed that an increase in interleukin- 4 in response to anti-inflammatory state was increased when the dose of toxin increased. This state might be due to the damaging effects of the toxins that impacted the body tissues.
Thus, it could be better explained in the cases where interleukin 4 and 10 significantly increased [17]. In one study the use of copper oxide nanoparticles with a dose of less than 50 nm resulted in reduction of superoxide dismutase and catalase. In one week following intrapulmonary injection, this damage was found to be increased [18] and consistent with the present study. In a study conducted by Lio et al (2011), copper oxide nanoparticles resulted in secretion of superoxide dismutase and catalase [19], which was similarly documented in our present study. Cellular oxidative stress resulted in an increased ROS level, decrease of GSH expression and increase of lipid peroxidation. The cells that were exposed to silica nanoparticles showed a significant wrinkling and condensation of nuclei, the signs of apoptosis. Different mechanisms are suggested for explaining damaging acts of nanoparticles among which the elevation of intracellular ROS level is more important. Superoxide, hydrogen peroxides, hydroxyls and other oxygen radicals can directly damage cell DNA, proteins, and lipids [20,21]. Reduction of GSH and production of ROS resulted in disharmony in the mitochondria function and some changes in gene expression, inflammation and apoptosis process including MAPK/EPK Kinase, MIP-2, caspase 3 and BC12. Therefore, the apoptosis induced by SiO2 first induces an increase in ROS. Decrease of GSH and damage in mitochondrial DNA increased gene expressions related to receptors and ligands that lead to cell death [22].
Therefore, it should be noted that nickel oxide nanoparticles could act as a stimulant that resulted in disturbed secretion of different enzymes by impacting enzyme release system or destructing target tissues. Cytokines, is known to balance body’s immune system. The present study could be used with cautions for people working in mines and related industries, centers producing nanomaterial, research laboratories and all those who are exposed to nanomaterial to reduce damages caused by nanoparticles.
Conclusion
The results of this study indicated that nickel oxide nanoparticles that resulted in significant increase in interleukin-4 level and interferon-Ɣ also reduced glutathione peroxidase, catalase and increased malondialdehyde in rats. Therefore, it could be concluded that nickel oxide nanoparticles that release free radicals also destroy tissues and caused damage to body’s immune system.
References
- Donaldson K, Stone V, Tran C, et al.. Nanotoxicology, Occupational and Environmental Medicine. 2004; 61: 727-728.
- Kim JA, Lee N, Kim BH, et al. Enhancement of neurite outgrowth in PC12 cells by iron oxide nanoparticles. Biomaterials. 2011; 32: 2871-2877.
- Singh N, Manshian B, Jenkins GJ, et al. NanoGenotoxicology: the DNA damaging potential of engineered nanomaterials. Biomaterials. 2009; 30: 3891-914.
- Trouiller B, Reliene R, Westbrook A, Solaimani P, Schiestl RH. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer research. 2009; 69(22): 8784-8789.
- Sikka SC, Rajasekaran M, Hellstrom WJ. Role of oxidative stress and antioxidants in male infertility. Journal of andrology. 1995; 16(6): 464-468.
- Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertility and sterility. 2003; 79(4): 829- 843.
- Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. American journal of physiology Heart and circulatory physiology. 2008; 294(2): H570-H578.
- Baumber J, Ball BA, Gravance CG, Medina V, Davies-Morel MC. The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J Androl. 2000; 21(6): 895-902.
- Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA damage and disease: induction, repair and significance. Mutation research. 2004; 567(1): 1-61.
- Florea AM, Splettstoesser F, Busselberg D. Arsenic trioxide (As2O3) induced calcium signals and cytotoxicity in two human cell lines: SY-5Y neuroblastoma and 293 embryonic kidney (HEK). Toxicology and applied pharmacology. 2007; 220(3): 292-301.
- Aebi H. Catalase in vitro. Methods in enzymology. 1984; 105: 121-126.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. The Journal of laboratory and clinical medicine. 1967; 70(1): 158-169.
- Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clinica chimica acta; international journal of clinical chemistry. 1978; 90(1): 37-43.
- Alluwaimi AM, Hussein Y. Diazinon immunotoxicity in mice: modulation of cytokines level and their gene expression. Toxicology. 2007; 236(1-2): 123-131.
- DeWitt JC, Peden-Adams MM, Keller JM, Germolec DR. Immunotoxicity of perfluorinated compounds: recent developments. Toxicologic pathology. 2012; 40(2): 300-311.
- Di Gioacchino M, Petrarca C, Lazzarin F, Di Giampaolo L, Sabbioni E, Boscolo P, et al. Immunotoxicity of nanoparticles. International journal of immunopathology and pharmacology. 2011;24(1 Suppl): 65S-71S.
- Riahi B, Rafatpanah H, Mahmoudi M, Memar B, Brook A, Tabasi N, et al. Immunotoxicity of paraquat after subacute exposure to mice. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2010; 48(6): 1627-1631.
- Reddy ARN, Colony RK, andhra Pradesh K, Kumar CP. Pulmonary Toxicity of Copper Oxide (CuO) Nanoparticles in Rats. J Med Sci. 2013; 13(7): 571- 577.
- Liu Z, Liu S, Ren G, Zhang T, Yang Z. Nano-CuO inhibited voltage-gated sodium current of hippocampal CA1 neurons via reactive oxygen species but independent from G-proteins pathway. Journal of applied toxicology : JAT. 2011; 31(5): 439-445.
- Wang F, Gao F, Lan M, Yuan H, Huang Y, Liu J. Oxidative stress contributes to silica nanoparticleinduced cytotoxicity in human embryonic kidney cells. Toxicology in vitro. 2009; 23(5): 808-815.
- Aitken RJ. Free radicals, lipid peroxidation and sperm function. Reproduction, fertility, and development. 1995; 7(4): 659-668.
- Huang DM, Hsiao JK, Chen YC, Chien LY, Yao M, Chen YK, et al. The promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticles. Biomaterials. 2009; 30(22): 3645-3651.