Research Article - Biomedical Research (2017) Volume 28, Issue 5
The therapeutic effects of etanercept-methotrexate combination on experimental spinal cord injury
Ali Riza Gezici, Semih Akar, Tulin Firat, Yasar Dagistan, Seckin Emre Cancan*, Aysel Kukner and Nezih OzkanDepartment of Neurosurgery, Abant Izzet Baysal University School of Medicine, Bolu, Turkey
- *Corresponding Author:
- Seckin Emre Cancan
Department of Neurosurgery
Abant Izzet Baysal University School of Medicine, Turkey
Accepted date: October 14, 2016
Abstract
Background: Experimental studies have demonstrated that neurons keep dying in an unrecoverable and non-regenerative pattern in following hours after primary mechanical injury to spinal cord. The cascade of events which is called secondary injury is composed of vascular impairment, oedema, ischemia, inflammation, exotoxicity, electrolyte imbalance, lipid peroxidation, free radicals, necrosis and apoptotic cell death.
Aims: With clinical and histopathological tests, this study investigated the therapeutic effects of etanercept-methotrexate combination which is an option in mono-therapy resistant rheumatological diseases; but this combination has not been used on recovery processes in clip compression Spinal Cord Injury (SCI) model yet.
Study Design: Forty Spraque-Dawley rats were divided into five groups: group 1 (Sham-control), group 2 (SCI+2 ml saline intramuscular), group 3 (SCI+1.25 mg/kg etanercept), group 4 (SCI+0.5 mg/kg methotrexate) and group 5 (SCI+1.25 mg/kg etanercept+0.5 mg/kg methotrexate).
Methods: Rats were evaluated 1st, 3rd, 5th and 10th days after SCI, clinically by Drummond and Moore scale, under light microscopy and by Tunel test; after sacrification on 10th day.
Results: Clinical and histopathological results of all treatment groups were found significantly better than the results of the trauma group; also no superiority in the monotherapy groups, over each other, was noted.
Conclusion: Combined-treatment group had a statistically significant better outcome in preventing apoptosis, but there was no difference according to the clinical results.
Keywords
Spinal cord injury, Etanercept, Methotrexate
Introduction
Traumatic Spinal Cord Injury especially (SCI) effects young group of individuals in developing countries; and creates serious sociological, economical and physiological destructions [1] Enhanced medical attendance can decrease patient morbidity and mortality rates but pharmacotherapy studies-to limit neuronal injury and to stimulate regeneration- still remain limited [2]. Pathophysiology of spinal cord injury initiates with primary mechanical injury which includes axonal, blood vessel and cell membrane damage. In primary mechanical injury not all axons routing through the cord are injured; there are still remaining intact axons passing over the site of injury [3]. Those intact axons, which have survived the injury, are significantly important in prognosis of the case, and they constitute the major target to all pharmacotherapy options. Experiments in animal models have demonstrated that even 5% of axons to survive the injury may lead to regain the neurological function or can preserve it [4,5].
First reaction of human body to an injury or an infection is particularly to be the inflammation. Although early inflammatory stages are not welcomed in neurotrauma they are accepted to be in favour later on [6]. In inflammatory response to SCI, neutrophils and microglial cells are known to be the first elements to attend the early phase of events, according to the results of the animal or human experiments [7-10]. Those pilot cells are seen in the first 12-24 hours and disappear about in 3-5 days [9]. The neutrophil accumulation and activation are steered by many cytokines such as TNF-α, IL-1 and IL-6 [11]. Neutrophils do accompany to the modulation of the secondary injury mechanisms via neutrophil proteases and reactive oxygen molecules [12]. Minutes or even hours after SCI, those cells are activated or transform into macrophages. Macrophages add more to the destructive effects by releasing pro-inflammatory cytokines, reactive oxygen radicals, nitrous oxide and proteases [13]. They lead many biological substrates to change in a pathological manner, like peroxidation of the lipoid components of the oxidative stress cells. Results of inflammation in early stages (ischemia, cell/tissue oedema, oxidative degradation, myelin degradation, necrosis and apoptotic changes, e.g.) may end up with an increase in the volume of the lesion [14]. Furthermore, they can give rise to formation of a glial scar tissue, more likely to create an infection protective environment; thence can hinder a successful regeneration [15]. When those processes are taken into account, depletion of neutrophils or depression of their functions may derive neuro-protection and neurological healing [16].
Etanercept inhibits TNF-α and neutrophil activation, and also blocks cell membrane receptors. Due to these effects Etanercept is used as a monotherapy agent in many rheumatological diseases and spinal cord injury models [17-22]. Methotrexate is also used as a monotherapy agent in autoimmune, rheumatological diseases and spinal cord injury modelled studies [23-26]. And efficient results have been reported for both agents individually. Also, in the literature Methotrexate-Etanercept combination is used successfully especially in cases with mono-therapy resistant rheumatic diseases [27,28]. But no study can be seen in the literature, regarding methotrexate-etanercept as a combination therapy in SCI. Hereby with this study of ours, we tried to figure out how a synergistic effect can occur by the combination of etanerceptmethotrexate verses solely use of each agent.
Material and Methods
Experimental groups
The study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85-23, revised 1996) and approved by the Institutional Animal Ethics Committee at Abant Izzet Baysal University. Forty female and adult Spraque Dawley rats were divided randomly into five groups, weighing 250-300 g, eight rats in each:
Group 1: Control-Sham (only laminectomy).
Group 2: Trauma (SCI, 2 ml saline intraperitoneally (i.p)).
Group 3: Trauma+Etanercept (SCI, single dose of 1.25 mg/kg etanercept, (Mustafa Nevzat, Istanbul, Turkey) injected i.p immediately after SCI, spinal cord samples removed 10 day after SCI).
Group 4: Trauma+MTX (SCI, single 0.5 mg/kg dose of MTX (ready-to-use sterile solution, Kocak Farma, Istanbul, Turkey) injected i.p immediately after SCI, spinal cord samples removed 10 day after SCI).
Group 5: Trauma+MTX/Etanercept (SCI, single 0.5 mg/kg dose of MTX [ready-to-use sterile solution, Kocak Farma, Istanbul, Turkey)+single dose of 1.25 mg/kg Etanercept injected i.p immediately after SCI, spinal cord samples removed 10 day after SCI).
Surgical procedure
Anaesthesia was achieved by intramuscular injection of 10 mg/kg xylazine (Bayer, Istanbul, Turkey) and 60 mg/kg ketamine hydrochloride (Parke, Istanbul, Turkey) before the surgery. With the rats in a prone position, a T6-T10 midline skin incision was made. Three-level laminectomies (T7-T9) were performed leaving the dura matter intact, and SCI was produced by extradural compression of the spinal cord using an aneurysm clip with a closing force of 24 g. After surgery, 1.0 cc of saline was administered subcutaneously to replace the blood volume loss. In all of the injured groups, the spinal cord was compressed for 1 min. Sham-injured animals were only subjected to laminectomy. At the end of day 10, all rats were sacrificed with deep anaesthesia.
Approximately 10 mm of spinal cord specimens from the level between T7 and T9 were obtained from each rat, and in the trauma groups the cord sample was divided 3 mm below the epicenter of the injury site. The samples from the lower levels of the spinal cord lesion (epicenter) were used for histological and immunohistological analyses.
Neurological evaluation
Grading of motor disturbance: After SCI the hind limb motor functions of the rats were evaluated once a day on days 1, 3, 5 and 10 by an independent observer, according to the Drummond and Moore scale [29]. A score from 0 to 4 was assigned to each animal as: 0=paraplegic with no evidence of lower extremity motor function; 1=poor lower extremity motor function, flicker of movement, weak antigravity movement only; 2=moderate lower extremity function with good antigravity strength but inability to draw legs under body and/or hop; 3=the ability to draw legs under body and hop, but not normally; 4=normal motor function.
Histological evaluation
Light microscopy: Spinal cord biopsies were taken on the 10th day. All the histological studies were performed in a blinded fashion. Extensity of the necrosis was evaluated in three main specimens which were collected 3 mm caudal to the epicenter and specimens were analysed for 4 criteria established for acute spinal cord injury by Black et al. [30], as: 1) White matter degeneration, characterized by oedema, formation of cysts, demyelination and infiltration of macrophages, cystic necrosis and cytoarchitectonic disorganization; 2) Haemorrhage in white or gray matter; 3) Neuronal loss, sometimes with vacuolization and inflammatory infiltration in gray matter; 4) Signs of hypoxic injury: nuclear retraction and pyknosis, as well as intense eosinophilic staining of the pericardium. Based on the described criteria, histological alterations related to the intensity of necrosis were classified semi-quantitatively into the following four categories after scanning all slices: <1% of total scanned area: score 0; 1-24% of total area: score 1; 25-49%: score 2; 50-74%: 3; and finally>75%: score 4. This quantification was performed by one independent and experienced pathologist on three different sections in a blinded manner.
Terminal deoxynucleotidyltransferase-mediated UTP end labelling assay (TUNEL)
TUNEL assay was conducted by using a TUNEL detection kit according to the manufacturer’s instruction (Apotag, HRP kit; DBA srl). The site of the trauma on the spinal cord was accepted as the "epicenter" and apoptotic cells in three different main sections, which were collected 3 mm caudal to the epicenter, were counted by an independent pathologist.
Statistical analysis
SPSS (Statistical Package for Social Sciences) for Windows 10.0 was used in the analysis. Since parameters had no regular distribution, Kruskal Wallis test was used in comparisons of quantitative data from groups. And Mann-Whitney U test was preferred to detect the group which causes the difference. When comparing the parameters within the groups, Wilcoxon signed rank test was used. Results were evaluated in a 95% confidence interval and the significance was accepted at the level of p<0.05.
Results
Reduction in the body weights of the subjects before and after the experiment was significant. After day 7 significant atrophy in the lower extremity muscles was observed in the trauma group, where as it was not prominent in the treatment groups. After the sacrification of the subjects, macroscopic oedema and haemorrhage was seen on extracted medulla spinalis structures.
Neurological results
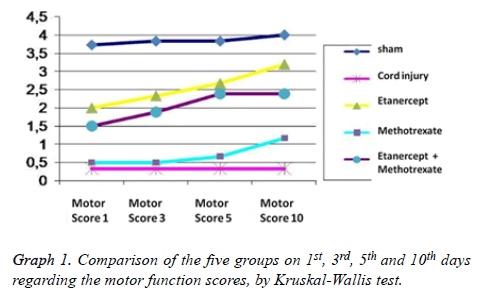
Grading of motor disturbance: On days 1, 3, 5 and 10 the difference regarding the motor function scores between all groups remains significant (p<0.05) (Table 1). In binary comparison of the groups regarding the 1st day motor function scores there is a significant difference between Sham group and all other groups; and between trauma group and all treatment groups. On the other hand when we look at the groups that have been administered medication, no difference was observed at all. When day 3 and day 5 motor function scores are taken into account it was seen that only the difference between the Sham group and group 3 (Etanercept) was lost (p: 0.181; p>0.05); but all other scores remained same as in the 1st day (Table 2). Also evaluation of the motor function scores from day 10 revealed that the difference between Sham group and group 3; and group 5 (Etanercept- Methotrexate) were lost (p: 0.329, p: 0.59; p>0.05); but all other scores remained the same as in day 1. According to the motor function scores, recovery rates in group 3 (Etanercept) and group 5 (etanercept-methotrexate) were highest and this finding was statistically significant. Also there was a significant difference between group 2 (trauma) and all other treatment groups, in favour of treatment groups regarding recovery. But there was no significant difference between the treatment groups in terms of recovery (Table 2, Graph 1).
| Groups | Sham | Trauma | Etanercept | Methotrexate | Etanercept+ Methotrexate |
Comparison of the groups by Kruskal-Wallis test | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | St error | Mean | St error | Mean | St error | Mean | St error | Mean | St error | P | |
| MS-1 | 3.83 | 0.167 | 0.33 | 0.21 | 2 | 0.63 | 0.5 | 0.5 | 1.5 | 0.5 | 0.04 |
| MS-3 | 3.83 | 0.167 | 0.33 | 0.21 | 2.33 | 0.76 | 0.5 | 0.5 | 1.88 | 0.64 | 0.005 |
| MS-5 | 3.83 | 0.167 | 0.33 | 0.21 | 2.67 | 0.71 | 0.67 | 0.49 | 2.38 | 0.56 | 0.002 |
| MS-10 | 4.0 | 0 | 0.33 | 0.51 | 3.2 | 1.34 | 1.17 | 1.16 | 2.38 | 1.76 | 0.04 |
Table 1. Comparison of the five groups on 1st, 3rd, 5th and 10th days regarding the motor function scores, by Kruskal-Wallis test (p<0.05).
| Groups | Motor score 1 | Motor score 3 | Motor score 5 | Motor score 10 |
|---|---|---|---|---|
| Group 1 vs. 2 | 0.002 | 0.002 | 0.002 | 0.002 |
| Group 1 vs. 3 | 0.006 | 0.07 | 0.181 | 0.329 |
| Group 1 vs. 4 | 0.002 | 0.002 | 0.003 | 0.002 |
| Group 1 vs. 5 | 0.021 | 0.026 | 0.03 | 0.059 |
| Group 2 vs. 3 | 0.041 | 0.03 | 0.028 | 0.009 |
| Group 2 vs. 4 | 0.043 | 0.045 | 0.042 | 0.024 |
| Group 2 vs. 5 | 0.031 | 0.012 | 0.03 | 0.048 |
| Group 3 vs. 4 | 0.093 | 0.071 | 0.44 | 0.36 |
| Group 3 vs. 5 | 0.783 | 0.685 | 0.546 | 0.435 |
| Group 4 vs. 5 | 0.2 | 0.098 | 0.066 | 0.228 |
Table 2. Binary comparison of the groups according to the motor scores by Mann-Whitney-U test (p<0.05).
Only in Group 3 (Ethanercept) a significant pattern of difference on motor function scores was seen when results from day 1 and 10 were compared by the Wilcoxon test applied inside the same groups (p<0.05). All other in-group analyses were not found to be significant (p>0.05).
Histological results
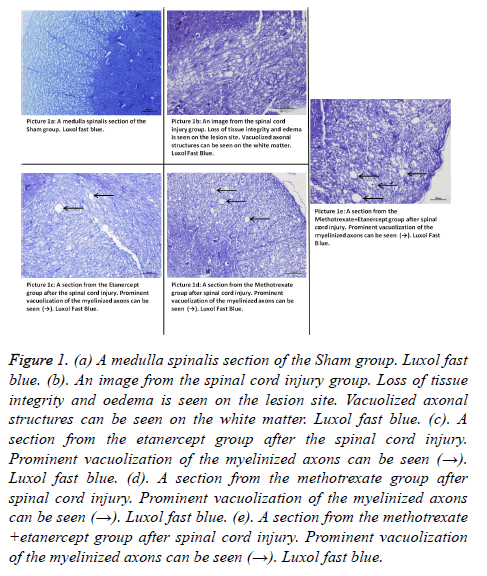
Light microscopy results: Luxol Fast Blue: Myelin structure was observed by Luxol Fast Blue staining. In sham group, myelin structure was clearly stained by Luxol Fast Blue in white matter. A significant loss of myelin was observed in injury group. Axonal swelling and vacuolization was significant in injury group. In contrast, less myelin loss was observed in Etanercept group. Axonal swelling and vacuolization was seen minimal in etanercept group and maximal in Etanercept-Methotrexate combined group. Methotrexate group was seen similar with injury group (Figures 1a-1e).
Figure 1. (a) A medulla spinalis section of the Sham group. Luxol fast blue. (b). An image from the spinal cord injury group. Loss of tissue integrity and oedema is seen on the lesion site. Vacuolized axonal structures can be seen on the white matter. Luxol fast blue. (c). A section from the etanercept group after the spinal cord injury. Prominent vacuolization of the myelinized axons can be seen (→). Luxol fast blue. (d). A section from the methotrexate group after spinal cord injury. Prominent vacuolization of the myelinized axons can be seen (→). Luxol fast blue. (e). A section from the methotrexate +etanercept group after spinal cord injury. Prominent vacuolization of the myelinized axons can be seen (→). Luxol fast blue.
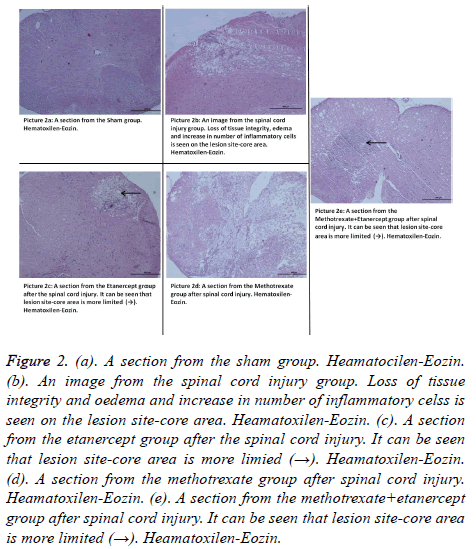
Hematoxylin-Eozin: According to the Haematoxylin-Eosin dye staining necrosis results on day 10, there was a significant difference between all groups (p: 0.00; p<0.05) (Table 3). There was a highly significant difference in binary comparison according to the necrosis scores, between Group 1 (control- Sham) and all other groups. When Group 2 was compared with other groups it was noted that all groups had statistically significance, in favour of treatment groups (p<0.05) (Table 4) (Figures 2a-2e). Controversially monotherapy groups had a significant difference in preventing necrosis when compared to the combination group (p: 0.001; p: 0.001; p<0.05) (Tables 3 and 4).
| Groups | Necrosis score | Apoptotic cells |
|---|---|---|
| Group 1 (sham) | 0 | 0.33+0.21 |
| Group 2 (Cord injury) | 2.33+0.21 | 11.33+1.33 |
| Group 3 (Etanercept) | 1.17+0.17 | 4.67+0.56 |
| Group 4 (Methotrexate) | 1.17+0.17 | 6.5+0.67 |
| Group 5 (Etanercept + Methotrexate) | 2.75+0.161 | 2.38+0.46 |
| p | 0.000 | 0.000 |
Table 3. Comparison of the results from necrosis and apoptosis scores by Kruskal-Wallis test, on the 10th day (p<0.05).
| Groups | Necrosis | Number of apoptotic cells |
|---|---|---|
| Group 1 vs. 2 | 0.002 | 0.003 |
| Group 1 vs. 3 | 0.001 | 0.003 |
| Group 1 vs. 4 | 0.001 | 0.003 |
| Group 1 vs. 5 | 0.001 | 0.011 |
| Group 2 vs. 3 | 0.006 | 0.004 |
| Group 2 vs. 4 | 0.006 | 0.01 |
| Group 2 vs. 5 | 0.013 | 0.002 |
| Group 3 vs. 4 | 1 | 0.5 |
| Group 3 vs. 5 | 0.001 | 0.008 |
| Group 4 vs. 5 | 0.001 | 0.002 |
Table 4. Binary comparison of the results from necrosis and apoptosis results of the groups on day 10, by Mann-Whitney-U test (p<0.05).
Figure 2. (a). A section from the sham group. Heamatocilen-Eozin. (b). An image from the spinal cord injury group. Loss of tissue integrity and oedema and increase in number of inflammatory celss is seen on the lesion site-core area. Heamatoxilen-Eozin. (c). A section from the etanercept group after the spinal cord injury. It can be seen that lesion site-core area is more limied (→). Heamatoxilen-Eozin. (d). A section from the methotrexate group after spinal cord injury. Heamatoxilen-Eozin. (e). A section from the methotrexate+etanercept group after spinal cord injury. It can be seen that lesion site-core area is more limited (→). Heamatoxilen-Eozin.
Terminal deoxynucleotidyltransferase-mediated UTP end labeling assay (TUNEL) results
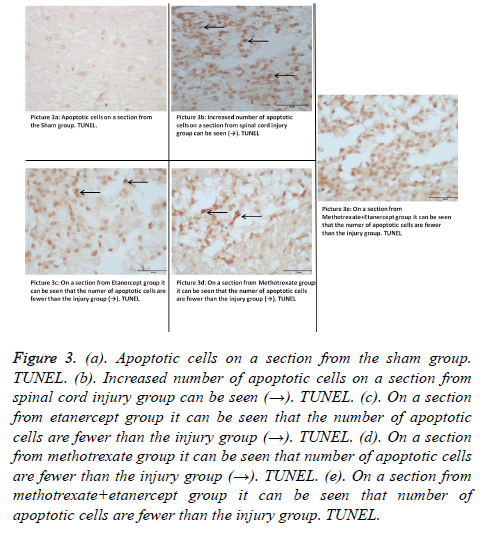
Outcomes of TUNEL on day 10, pointed out that there was a statistical difference in between all groups (p: 0.00; p<0.05) (Table 3). When number apoptotic cells were compared in binary groups on day 10, a significant difference between Group 1 and all other groups was seen. Also when trauma and monotherapy groups were compared, a significant difference in favour of monotherapy groups was seen. But no statistical significance has been shown in Group 3 (Etanercept) verses Group 4 (Methotrexate) (p: 0.05; p>0.05) (Table 4) (Figures 3a-3e). In preventing the apoptosis etanercept-methotrexate combination had a significant difference when compared with the monotherapy groups (p: 0.008; p: 0.002; p<0.05) (Tables 3 and 4).
Figure 3. (a). Apoptotic cells on a section from the sham group. TUNEL. (b). Increased number of apoptotic cells on a section from spinal cord injury group can be seen (→). TUNEL. (c). On a section from etanercept group it can be seen that the number of apoptotic cells are fewer than the injury group (→). TUNEL. (d). On a section from methotrexate group it can be seen that number of apoptotic cells are fewer than the injury group (→). TUNEL. (e). On a section from methotrexate+etanercept group it can be seen that number of apoptotic cells are fewer than the injury group. TUNEL.
Discussion
Studies have proved that reproduction of macrophages and fibroblasts, their differentiations and functions are inhibited by glucocorticoids which influence production and release of IL-1, IL-6 and TNF-α [31-33]. Then animal studies to take the results further, by proving that antagonizing TNF-α is a more convenient treatment strategy, were also concluded [18]. At the site of injury one hour after the SCI an important increase in number of receptors, synthesis and secretion of TNF-α and IL-1 is detected [34,35]. In animal study models anti TNF-α treatment is reported as an efficient treatment strategy [18]. TNF-α has some significant roles in other pathologies like rheumatoid arthritis; and inhibiting TNF-α provides improvement in the symptoms of rheumatoid arthritis [19].
The main target to use a TNF-α antagonist is to eliminate the excess levels of TNF-α in the circulation and at the site of inflammation. Etanercept competitively inhibits TNF-α activity and blocks interaction with cell membrane receptors. We also see that etanercept is used to treat different diseases because of its anti-inflammatory effects. There are several mono and combination treatment trials on arthritic rats, proving its antiinflammatory effectiveness. There are several animal trials of monomeric PEGylated type-I TNFR (PEG TNFRI), expressing its effectiveness or its synergistic positive results together with methotrexate, dexmedetomidine or indomethacin on rats with arthritis [36,37]. Also different trials on animals showed Etanercept to be effective in spinal cord injury. Genovese et al. figured out that post-inflammatory reaction is reduced and motor functions are improved with etanercept treatment at the dose of 5 mg/kg after SCI; the same group of investigators also pointed out the advancement of this positive result with etanercept-dexamethasone combination [17,20,21]. Additionally Etanercept treatment is showed to improve neurological healing especially in the acute phase by Chi et al. [22]; Chen et al. also showed it to reduce oligodendroglial and neuronal apoptosis, and to fix extremity's locomotor function, increase myelinisation [38]; as well Fatih et al. pointed out the efficacy of Etanercept on clinical and neurophysiologic recovery in partial spinal cord injury model [39]. In our study when we look at the binary comparison of the groups (Mann- Whitney U test), the recovery data of the spinal trauma group, which underwent etanercept treatment, is significantly superior verses trauma group; on both clinical (motor scores) and histological bases (necrosis-apoptosis score). And this result clearly endorses the neuro-protective effect of etanercept with other trials in the literature [17,20-22,38-41].
Methotrexate (MTX) is being used at low doses due to its indirect immune suppressive effects in auto-immune diseases such as Rheumatoid Arthritis (RA), psoriasis and allograft reaction [23-25]. But high-dose MTX has anti-tumor activity in different types of neoplasms; and is used to treat hydatiform mole, meningeal leukaemia, advance-staged non-Hodgkin lymphomas and other malignancies [42]. MTX may also have additional effects on secondary injury mechanisms by such routes of action like repressing purine/pyrimidine synthesis or lymphocyte proliferation, neutrophil chemo taxis and adherence; and provocating adenosine release, activating T-cell apoptosis and decreasing blood immunoglobulin levels thus inhibiting pro-inflammatory cytokine production [43]. Secondary end point of this study is to expose the efficacy of low-dose MTX treatment in preventing secondary injuries after SCI. After a wide literature survey, we only came up with the results of Sanli et al. experimental study for MTX's effects on spinal cord injury [26]. According to this study MTX has a relation with a decrease in early phase neutrophil infiltration and correlated peroxidation of lipids. Therefore Sanli et al. study claimed that MTX has an important neuro-protective effect in the first 24-hour of the trauma. In our study, when we look at the binary comparison of the groups (Mann-Whitney U test) from the first day on, MTX group has a significant difference in favour; both in clinical score (motor score) and histopathologically. This result of our study is supportive to Sanli et al. trial; and claims Methotrexate to be neuroprotective [26].
On the other hand if we have a look at the binary comparison of Methotrexate with Etanercept in our study groups (Mann- Whitney U test), there is no significant difference both in clinical (motor scores) and histopathological (necrosisapoptosis scores) aspects. Because of the intensity and complexity of the inflammation process after SCI; targeting only one point of the secondary injury cascade results with failure in complete inhibition of the inflammation. Therapeutic outcome of the concurrent application of methotrexateetanercept combination in SCI could not be assessed previously. And this study is designed to understand if etanercept-methotrexate combination in SCI; can result in a synergistic effect, or not. In our study according to the clinically evaluated motor score parameters, a significant difference in favour of combination treatment group, was observed between the trauma group and the combination group. Regarding groups of methotrexate stand alone, etanercept stand alone, and in methotrexate-etanercept combination group; there is no significant difference in motor scores. According to the histopathological evaluations, only the apoptosis scores were found to be statistically low in favour of combination group. Controversially the necrosis scores were very high in the combination group when compared with the monotherapies.
Conclusion
We see that methotrexate-etanercept combination can only depress apoptosis rates in a statistically significant manner whereas this significance is not sufficient enough to carry out the statistical difference in clinical results, not better than the mono-therapies.
Conflict of Interest Declaration
All authors hereby declare that they have no conflicts of interest.
References
- Lee BB, Cripps RA, Fitzharris M, Wing PC. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord 2014; 52: 110-116.
- Impellizzeri D, Esposito E, Mazzon E, Paterniti I, Di Paola R, Bramanti P. The effects of a polyphenol present in olive oil, oleuropein aglycone, in an experimental model of spinal cord injury in mice. Biochem Pharmacol 2012; 83: 1413-1426.
- McDonald JW, Belegu V. Demyelination and remyelination after spinal cord injury. J Neurotrauma 2006; 23: 345-359.
- Fehlings MG. Summary statement: the use of methylprednisolone in acute spinal cord injury. Spine 2001; 26: S55.
- Kakulas BA. Neuropathology: the foundation for new treatments in spinal cord injury. Spinal Cord 2004; 42: 549-563.
- Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord 2003; 41: 369-378.
- Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 2010; 133: 433-447.
- Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD. The cellular inflammatory response in human spinal cords after injury. Brain 2006; 129: 3249-3269.
- Hamada Y, Ikata T, Katoh S, Nakauchi K, Niwa M, Kawai Y. Involvement of an intercellular adhesion molecule 1-dependent pathway in the pathogenesis of secondary changes after spinal cord injury in rats. J Neurochem 1996; 66: 1525-1531.
- McTigue DM, Tani M, Krivacic K, Chernosky A, Kelner GS. Selective chemokine mRNA accumulation in the rat spinal cord after contusion injury. J Neurosci Res 1998; 53: 368-376.
- Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Compar Neurol 2007; 500: 267-285.
- Bethea JR, Dietrich WD. Targeting the host inflammatory response in traumatic spinal cord injury. Curr Opin Neurol 2002; 15: 355-360.
- Bethea JR, Castro M, Keane RW, Lee TT, Dietrich WD. Traumatic spinal cord injury induces nuclear factor-kappaB activation. J Neurosci 1998; 18: 3251-3260.
- Conti A, Cardali S, Genovese T, Di Paola R, La Rosa G. Role of inflammation in the secondary injury following experimental spinal cord trauma. J Neurosurg Sci 2003; 47: 89.
- Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol 2008; 209: 294-301.
- Taoka Y, Okajima K, Uchiba M, Murakami K, Kushimoto S. Role of neutrophils in spinal cord injury in the rat. Neuroscience 1997; 79: 1177-1182.
- Genovese T, Mazzon E, Crisafulli C, Esposito E, Di Paola R. Combination of dexamethasone and etanercept reduces secondary damage in experimental spinal cord trauma. Neurosci 2007; 150: 168-181.
- Kapadia S, Torre-Amione G, Yokoyama T, Mann DL. Soluble TNF binding proteins modulate the negative inotropic properties of TNF-alpha in vitro. American Journal of Physiol Heart Circul Physiol 1995; 268: H517-H25.
- Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med 1996; 334: 1717-1725.
- Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muia C, Bramanti P. Immunomodulatory effects of etanercept in an experimental model of spinal cord injury. J Pharmacol Exp Therap 2006; 316: 1006-1016.
- Bendele A, McComb J, Gould T, Frazier J, Chlipala E, Seely J. Combination benefit of PEGylated soluble tumor necrosis factor receptor type I (PEG sTNF-RI) and dexamethasone or indomethacin in adjuvant arthritic rats. Inflam Res 1999; 48: 453-460.
- Chi L, Yu J, Zhu H, Li X, Zhu S, Li Z. Dual neuronal response to tumor necrosis factor-alpha following spinal cord injury. Neur Regen Res 2010; 5: 917-926.
- Genestier L, Paillot R, Quemeneur L, Izeradjene K, Revillard JP. Mechanisms of action of methotrexate. Immunopharmacol 2000; 47: 247-257.
- Hildner K, Finotto S, Becker C, Schlaak J, Schirmacher P, Galle P. Tumour necrosis factor (TNF) production by T cell receptor-primed T lymphocytes is a target for low dose methotrexale in rheumatoid arthritis. Clin Exp Immunol 1999; 118: 137-146.
- Neurath M, Hildner K, Becker C, Schlaak J, Barbulescu K, Germann T. Methotrexate specifically modulates cytokine production by T cells and macrophages in murine collagen-induced arthritis (CIA): a mechanism for methotrexate-mediated immunosuppression. Clin Exp Immunol 1999; 115: 42.
- Sanli AM, Serbes G, Sargon MF, Caliskan M, Kilinc K, Bult H. Methothrexate attenuates early neutrophil infiltration and the associated lipid peroxidation in the injured spinal cord but does not induce neurotoxicity in the uninjured spinal cord in rats. Acta Neurochirurgica 2012; 154: 1045-1054.
- Schulze-Koops H, Deeg M, Runge C, Volmer T, Brecht J. Bewertung der kombinations-therapie der rheumatoiden arthritis mit methotrexat und etanercept auf der basis der TEMPO-studie. Z Rheumatol 2009; 68: 836-841.
- Schnabel A, Gross W. Evidence-based treatment of methotrexate-resistant rheumatoid arthritis. Cytokine antagonists. Deutsche Med Wochens 2003; 128: 201-203.
- Drummond JC, Moore SS. The influence of dextrose administration on neurologic outcome after temporary spinal cord ischemia in the rabbit. Anesthesiol 1989; 70: 64-70.
- Black P, Markowitz RS, Cooper V, Mechanic A, Kushner H. Models of spinal cord injury static load technique. Neurosurgery 1986; 19: 752-762.
- Gonzalez R, Glaser J, Liu MT, Lane TE, Keirstead HS. Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp Neurol 2003; 184: 456-463.
- Gonzalez S, Labombarda F, Deniselle MaCG, Saravia FE, Roig P, De Nicola AF. Glucocorticoid effects on Fos immunoreactivity and NADPH-diaphorase histochemical staining following spinal cord injury. Brain Res 2001; 912: 144-153.
- Hurlbert RJ. Strategies of medical intervention in the management of acute spinal cord injury. Spine 2006; 31: S16-S21.
- Harrington JF, Messier AA, Levine A, Szmydynger-Chodobska J, Chodobski A. Shedding of tumor necrosis factor type 1 receptor after experimental spinal cord injury. J Neurotrauma 2005; 22: 919-928.
- Hayashi M, Ueyama T, Nemoto K, Tamaki T, Senba E. Sequential mRNA expression for immediate early genes, cytokines, and neurotrophins in spinal cord injury. J Neurotrauma 2000; 17: 203-218.
- McComb J, Gould T, Chlipala E, Sennelo G, Frazier J, Kieft G. Antiarthritic activity of soluble tumor necrosis factor receptor type I forms in adjuvant arthritis: correlation of plasma levels with efficacy. J Rheumatol 1999; 26: 1347-1351.
- Bendele A, McComb J, Gould T, Frazier J, Chlipala E, Seely J. Effects of PEGylated soluble tumor necrosis factor receptor type I (PEG sTNF-RI) alone and in combination with methotrexate in adjuvant arthritic rats. Clin Exp Rheumatol 1998; 17: 553-560.
- Chen KB, Uchida K, Nakajima H, Yayama T, Hirai T, Watanabe S. Tumor necrosis factor-a antagonist reduces apoptosis of neurons and oligodendroglia in rat spinal cord injury. Spine 2011; 36: 1350-1358.
- Bayrakli F, Balaban H, Ozum U, Duger C, Topaktas S, Kars HZ. Etanercept treatment enhances clinical and neuroelectrophysiological recovery in partial spinal cord injury. European Spine J 2012; 21: 2588-2593.
- Chio CC, Lin JW, Chang MW, Wang CC, Kuo JR. Therapeutic evaluation of etanercept in a model of traumatic brain injury. J Neurochem 2010; 115: 921-929.
- Dinomais M, Stana L, Egon G, Richard I, Menei P. Significant recovery of motor function in a patient with complete T7 paraplegia receiving etanercept. J Rehab Med 2009; 41: 286-288.
- Jolivet J, Cowan KH, Curt GA, Clendeninn NJ, Chabner BA. The pharmacology and clinical use of methotrexate. N Engl J Med 1983; 309: 1094-1104.
- Xinqiang S, Fei L, Nan L, Yuan L, Fang Y, Hong X. Therapeutic efficacy of experimental rheumatoid arthritis with low-dose methotrexate by increasing partially CD4+ CD25+ Treg cells and inducing Th1 to Th2 shift in both cells and cytokines. Biomed Pharmacotherap 2010; 64: 463-471.