Research Article - Biomedical Research (2017) Volume 28, Issue 8
The therapeutic effects of cyclosporin-A on experimental spinal cord injury
Ali Riza Gezici1, Guven Kilic1, Tulin Firat2, Seckin Emre Cancan1*, Aysel Kukner2, Nezih Ozkan1 and Yasar Dagistan1
1Department of Neurosurgery, School of Medicine, Abant Izzet Baysal University, Bolu-Turkey
2Department of Histology and Embriyology, School of Medicine, Abant Izzet Baysal University, Bolu-Turkey.
- *Corresponding Author:
- Seckin Emre Cancan
Department of Neurosurgery
Abant Izzet Baysal University School of Medicine, Bolu, Turkey
Accepted date: February 03, 2017
Abstract
Background: According to the experiments, neutrophils and microglial cells are the first to attend the early phase of events in inflammatory response to SCI. Those pilot cells are seen in the first 12-24 hours and disappear about 3-5 days. The neutrophil accumulation and activation are steered by many cytokines such as TNF-α, IL-1 and IL-6. Neutrophils do accompany to the modulation of secondary injury mechanisms via neutrophil proteases and reactive oxygen molecules. When those processes are taken into account, depletion of neutrophils or depression of their functions may derive neuro-protection and neurological healing.
Purpose: To investigate the therapeutic and neuroprotective effects of Cyclosporin-A (CSA) on recovery processes using clinical and histopathological tests, which has not been used very frequently in clip compression spinal cord injury (SCI) models.
Material and Methods: Twenty-four Spraque-Dawley rats were divided into three groups: group 1 [Sham-control, n=8], group 2 [SCI+2 mL saline intramuscular (i.m.), n=8], group 3 [SCI+5 mg/kg CSA (i.p.) 1 h after SCI and for the following three days, n=8]. Rats were evaluated 1st, 3rd, 5th and 10th days after SCI, clinically by Drummond and Moore scale and under light microscopy and by TUNEL test; after scarification on 10th day.
Results: Clinical and histopathological results of treatment group were found significantly better than the results of the trauma group.
Conclusion: CSA can depress apoptosis and necrosis rates in a statistically significant manner and carry out the statistical difference in clinical results.
Keywords
Animals, Cyclosporine, Immunosuppressive agents, Motor activity, Spinal cord injuries, Thoracic vertebrae.
Introduction
First reaction of body to injury and infections is particularly the inflammation. The role of inflammation after spinal cord injury (SCI) is defined in details; but profitable and destructive effects are still debatable. In general aspect, although early stage inflammatory events are not welcomed in neurotrauma, they are thought to be in favour on late stages [1]. According to the experiments on animals and humans, neutrophils and microglial cells are the first to attend the early phase of events in inflammatory response to SCI [2-5]. Those pilot cells are seen in the first 12-24 hours and disappear about in 3-5 days [1]. The neutrophil accumulation and activation are steered by many cytokines such as TNF-α, IL-1 and IL-6 [6]. Neutrophils do accompany to the modulation of secondary injury mechanisms via neutrophil proteases and reactive oxygen molecules [7]. Minutes or even hours after SCI, those cells are activated or transform into macrophages. Macrophages add more to the destructive effects by releasing pro-inflammatory cytokines, reactive oxygen radicals, nitrous oxide and proteases [8]. They lead many biological substrates to change in a pathological manner, such as peroxidation of the lipoid components of the oxidative stress cells. The results of an early staged inflammation; like ischemia, cell/tissue edema, oxidative degradation, myelin degradation, necrosis and apoptotic changes, may increase the volume of the lesion [9]. Furthermore, those changes give rise to glial scar tissue and development of the infection protective environment; thence hinder creation of a successful regeneration [10]. When those processes are taken into account, depletion of neutrophils or depression of their functions may derive neuro-protection and neurological healing [11].
This study aims to investigate the protective (neuroprotective) and therapeutic (regenerative) effects of the cyclosporin-A, which is an immunosuppressive drug, on secondary spinal cord injury in a rat spinal injury model; in structural and also functional aspects.
Materials and Methods
Experimental groups
The investigation was conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85-23, revised 1996) and approval was received from the Institutional Animal Ethics Committee at Abant ?zzet Baysal University. Twenty four female and adult Spraque Dawley rats, weighing 250-300 g, were divided randomly into three groups; composed of eight rats in each:
Group 1: Control-Sham (only laminectomy, no SCI, n=8)
Group 2: Trauma (SCI, 2 mL saline intraperitoneally [i.p], n=8)
Group 3: Trauma+CSA (SCI, single 5 mg/kg syclosporin-A [Sandimmun Novartis] injected i.p. immediately after SCI and for the following three days, once a day, n=8)
All rats were evaluated on 1st, 3rd, 5th and 10th days after SCI, clinically by Drummond and Moore scale, under light microscopy and by TUNEL test; after sacrification on day 10.
Surgical procedure
The rats were anesthetized with intramuscular injection of 10 mg/kg xylazine (Bayer, Istanbul, Turkey) and 60 mg/kg ketamine hydrochloride (Parke Davis, Istanbul, Turkey) before surgery. With the rats in a prone position, a T6-T10 midline skin incision was made. Three-level laminectomies (T7-T9) were performed, leaving the dura matter intact, and SCI was produced by extradural compression of the spinal cord using an aneurysm clip with a closing force of 24 g. In all of the injured groups, the spinal cord was compressed for 1 min. Shaminjured animals were only subjected to laminectomy. After surgery, 2.0 cc of saline was administered intraperitoneally to replace the blood volume lost during the surgery. During recovery from anaesthesia, the rats were placed on a warm heating pad and covered with a warm towel. The rats were singly housed in a temperature-controlled room at 27°C for a survival period of 10 days. Food and water were provided to the rat ad libitum. During this time period, the bladders of the animals were manually voided twice a day until the mice were able to regain normal bladder function. At the end of day 10, all rats were killed under deep anaesthesia.
Approximately 10 mm of spinal cord from the level between T7 and T9 was obtained from each rat, and in the trauma groups the cord sample was divided 3 mm below the epicenter of the injury. The samples from the lower levels of the spinal cord lesion (epicenter) were used for histological and immunohistological analyses.
Neurological evaluation
Grading of motor disturbance: The hind limb motor function of rats were evaluated once a day on days 1, 3, 5 and 10 after SCI by an independent observer according to the Drummond and Moore scale [12]. A score of 0 to 4 was assigned to each animal as follows: 0=paraplegic with no evident lower extremity motor function; 1=poor lower extremity motor function, flicker of movement, weak antigravity movement only; 2=moderate lower extremity function with good antigravity strength but inability to draw legs under body and/or hop; 3=the ability to draw legs under body and hop, but not normally; 4=normal motor function.
Histological evaluation
Light microscopy: Spinal cord biopsies were taken on 10th day. The biopsies were fixed for 24 h in paraformaldehyde solution (4% in 0.1 M PBS) at room temperature, dehydrated by graded ethanol, and embedded in paraplast (Sherwood Medical, Mahwah, NJ). Tissue sections (thickness 5_m) were deparaffinised with xylene, stained with H&E and studied using light microscopy (Dialux 22 Leitz; DBA srl). All the histological studies were performed in a blinded fashion. Extensity of the necrosis was evaluated in three main specimens which were collected 3 mm caudal to the epicenter and specimens were analyzed for 4 criteria established for acute spinal cord injury by Black et al. [13], as follows: 1- white matter degeneration, characterized by edema, formation of cysts, demyelination and infiltration of macrophages, cystic necrosis and cytoarchitectonic disorganization. 2-hemorrhage in white or gray matter; 3-neuronal loss, sometimes with vacuolization and inflammatory infiltration in gray matter; 4- signs of hypoxic injury: nuclear retraction and pyknosis, as well as intense eosinophilic staining of the pericardium. Based on the described criteria, histological alterations related to the intensity of necrosis were classified semi-quantitatively into the following four categories after scanning of all slices; <1% of total scanned area: score 0; 1-24% of total area: score 1; 25-49%: score 2; 50-74%: 3; and finally >75%: score 4. This quantification was performed by one independent and experienced pathologist on three different sections in a blinded manner and without knowledge of the experimental group.
Terminal Deoxynucleotidyltransferase-Mediated UTP end labelling assay (TUNEL)
TUNEL assay was conducted by using a TUNEL detection kit according to the manufacturer’s instruction (Apotag, HRP kit; DBA srl). In brief, sections were incubated with 15 g/ml proteinase K for 15 min at room temperature and then washed with PBS. Endogenous peroxidase was inactivated by 3% H2O2 for 5 min at room temperature and then washed with PBS. Sections were immersed in TdT buffer containing deoxynucleotidyl transferase and biotinylated UTP in TdT buffer, incubated in a humid atmosphere at 37°C for 90 min, and then washed with PBS. The sections were incubated at room temperature for 30 min with anti-horseradish peroxidaseconjugated antibody, and the signals were visualized with diaminobenzidine. The site of the trauma on the spinal cord was accepted as the "epicenter" and apoptotic cells in three different main sections which were collected 3mm caudal to the epicenter, were counted.
Statistical analysis
SPSS (Statistical Package for Social Sciences) for Windows 10.0 was used in the analysis. Since parameters had no regular distribution, Kruskal Wallis test was used in comparisons of quantitative data from groups. And Mann-Whitney U test was preferred to detect the group which causes the difference. When comparing the parameters within the groups, Wilcoxon signed rank test was used. Results were evaluated in a 95% confidence interval and the significance was accepted at the level of p<0.05.
Results
Reduction in the body weights of the subjects before and after the experiment was significant. After day 7, significant atrophy in the lower extremity muscles was observed in the trauma group whereas it was not prominent in the treatment group. After the sacrification of the subjects, macroscopic edema and hemorrhage was seen on extracted medulla spinalis structures.
Neurological results
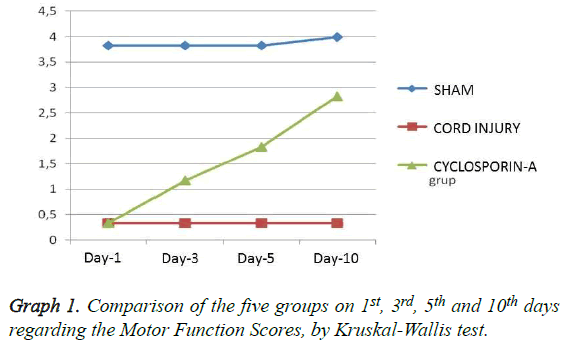
Grading of motor disturbance: On days 1, 3, 5 and 10 the difference regarding the motor function scores between all groups remains significant (p<0.05) (Table 1). When binary comparisons of the groups were evaluated it has been seen that in duration of the whole test, group 2 (trauma) and group 3 (CSA) were not able to reach the scores of the SHAM group regarding the motor scores (p<0.05). But starting from day 5, a significant difference was observed in favour of group 3 when group 2 was compared with group 3 (p:0.002) and this difference got more prominent on day 10 (p:0.001) (Table 2 and Graph 1). According to the in-group analysis of the groups with Wilcoxon test, only group 3 (CSA) had significant difference regarding the day 1 and 10 comparisons (Table 3).
| Groups | Sham | Trauma | Cyclopsorin-A | Comparison of the groups by Kruskal-Wallis test | |||
|---|---|---|---|---|---|---|---|
| mean | St error | mean | St error | mean | St error | p | |
| MS-1 | 3.83 | 0.167 | 0.33 | 0.211 | 0.33 | 0.211 | 0.002 |
| MS-3 | 3.83 | 0.167 | 0.33 | 0.211 | 1.17 | 0.307 | 0.001 |
| MS-5 | 3.83 | 0.167 | 0.33 | 0.211 | 1.83 | 0.167 | 0.000 |
| MS-10 | 4 | 0 | 0.33 | 0.211 | 2.83 | 0.167 | 0.000 |
Table 1: Comparison of the three groups on 1st, 3rd, 5th and 10th days regarding the Motor Function Scores, by Kruskal-Wallis test (p<0.05).
| Groups | Motor score 1 | Motor score 3 | Motor score 5 | Motor score 10 | Necrosis | Number Apoptotic cells |
|---|---|---|---|---|---|---|
| Groups 1, 2 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.003 |
| Groups 1, 3 | 0.002 | 0.003 | 0.002 | 0.001 | 0.002 | 0.003 |
| Groups 2, 3 | 1 | 0.057 | 0.004 | 0.002 | 0.014 | 0.008 |
Table 2: In-group comparison of the Motor Score data by Wilcoxon test.
| Group | Motor Score 3- Motor Score 1 | Motor Score 5 - Motor Score 1 | Motor Score 10 - Motor Score 1 | Motor Score 5 - Motor Score 3 | Motor Score 10 - Motor Score 3 | Motor Score 10 - Motor Score 5 | |
|---|---|---|---|---|---|---|---|
| SHAM | Z Asymp. Sig. (2-tailed) |
.000b | .000b | -1.000c | .000b | -1.000c | -1.000c |
| 1.000 | 1.000 | .317 | 1.000 | .317 | .317 | ||
| Cord injury | Z Asymp. Sig. (2-tailed) |
.000b | .000b | .000b | .000b | .000b | .000b |
| 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | ||
| Cord injury+Cyclosporin-A | Z Asymp. Sig. (2-tailed) |
-2.236c | -2.251c | -2.251c | -2.000c | -2.271c | -2.449c |
| .025 | .024 | .024 | .046 | .023 | .014 | ||
Table 3: In-group comparison of the Motor Score data by Wilcoxon test.
Histological results
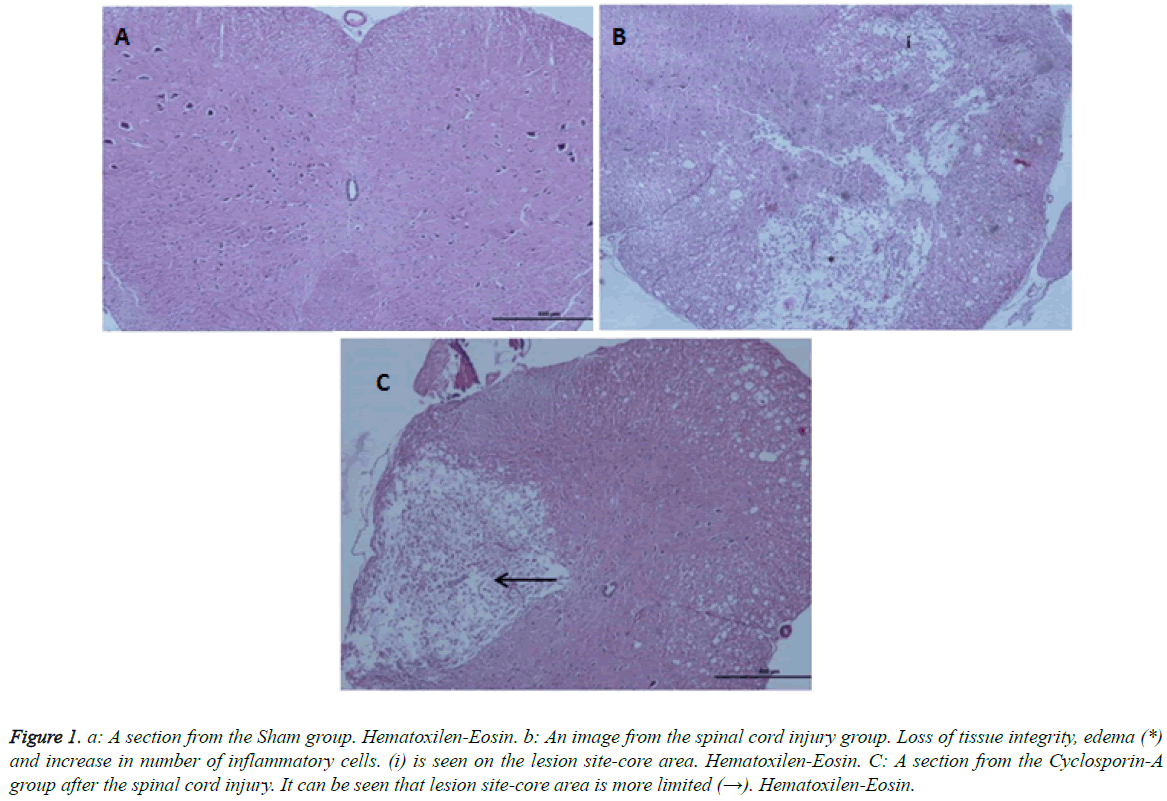
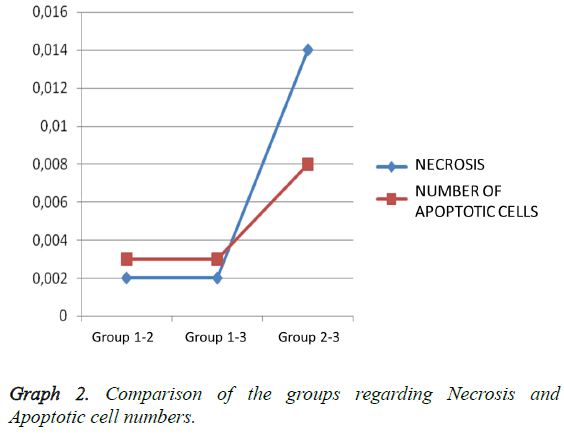
Light microscopy results: According to the Hemotoxylin-Eosin dye staining necrosis results on day 10, there was a significant difference between all groups (p: 0.00; p<0.01) (Table 2). It was seen that there is a high level of significant difference in binary comparison of the groups according to the necrosis scores, between Group 1 (control-Sham) and other groups (Figure 1). When Group 2 was compared with group 3 (CSA) it was noted that groups had statistically significance difference, in favor of treatment group (p: 0.008) (Table 2; Graph 2). Briefly cyclosporin-A causes a statistical difference in prevention of necrosis when compared with the trauma group.
Figure 1: a: A section from the Sham group. Hematoxilen-Eosin. b: An image from the spinal cord injury group. Loss of tissue integrity, edema (*) and increase in number of inflammatory cells. (i) is seen on the lesion site-core area. Hematoxilen-Eosin. C: A section from the Cyclosporin-A group after the spinal cord injury. It can be seen that lesion site-core area is more limited (→). Hematoxilen-Eosin.
Terminal deoxynucleotidyltransferase-Mediated UTP end labeling assay (TUNEL) results
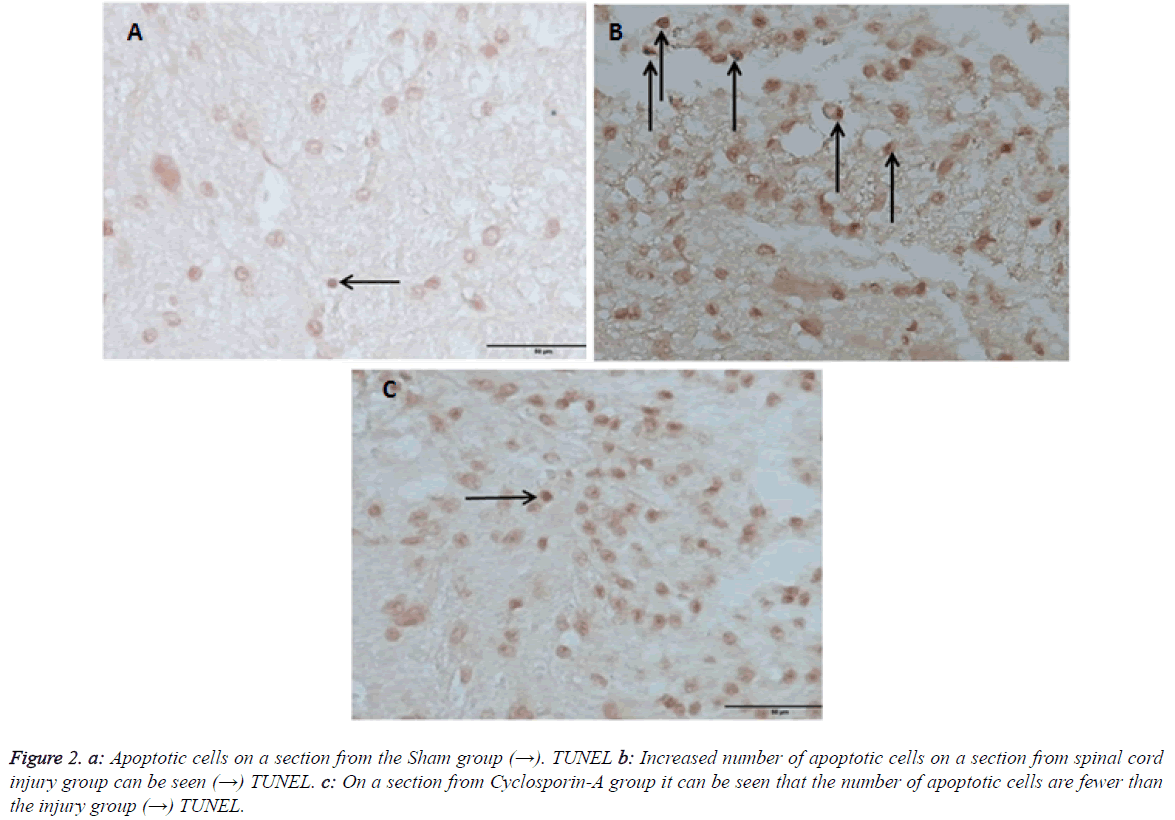
Outcomes of TUNEL on day 10 pointed out that there was a statistical difference in between all groups (Table 2). On day 10 comparing the number apoptosis in binary groups, a significant difference between Group 1 and other groups was seen (Figure 2). Also when group 2 (trauma) and group 3 (CSA) are compared a significant statistical difference in favor of therapy group is seen (p: 0.008) (Table 2 and Graph 2). In preventing the apoptosis Cyclosporin-A has a significant difference when compared with the trauma group.
Figure 2: Apoptotic cells on a section from the Sham group (→). TUNEL b: Increased number of apoptotic cells on a section from spinal cord injury group can be seen (→) TUNEL. c: On a section from Cyclosporin-A group it can be seen that the number of apoptotic cells are fewer than the injury group (→) TUNEL.
Discussion
Immunosuppresive agents can be divided into four major groups: 1. T-cell blockers (Cyclosporin-A, Tacrolimus (FK506), Sirolimus), 2. Glucorticoids, 3. Cytotoxic drugs, 4. Anticore Markers (reagent). As a T-cell blocker, Cyclosporin-A is the most frequently and effectively used agent as an immunosuppresive drug. Cyclosporin-A is a cyclicpolipeptide, composed of 11 amino acids and derived from a fungus named as Tolypocladiuminflatum Gams. Following the recognition of the antigen, cyclosporin-A creates immunosuppression by preventing the early stages of the autoimmune response. It cannot treat an autoimmune response, which occured previously, or an occured rejection reaction; but it may prevent a reponse or reaction if it is given prior to occurence. It selectively inhibits inductor/helper subtype T lymphocytes (CD4+) and blocks their proliferation and differentiation [14]. It has no effect on the mature T cells. So it should be used as soon as possible after the encounter with an antigen (for example in an allogenictransplantation), preferentially in the first 24 hours [14]. Therefore we have admistered the treatment right after the trauma. It has its immunosuppresive and secondary neuronal injury preventive effect on CD4+ T lymphocytes by inhibiting calcineurin, which is a Ca++ depended enzyme and one of the steps of the signalling cascade that is initiated by stimulation of the T lymphocyte receptor with antigen. In this chain of reactions, cyclosporin-A inhibits Ca++ transportation via mitochondria membrane trasportation (MMT) which is located on the mitochondria membrane. In order to have this effect cyclosporin-A should bind to cyclophlin, which is a cytoplasmic receptor. Binding of cyclosporin-A to cyclophilin causes a protein complex which inhibits activation of calcium/calmodulin dependent calcineurin.This complex inhibits calcineurin or protein phosphatase 2B [14]. If this calcineurin is not inhibited it causes dephosphorilation of nuclear factor of activated T-cells (NTF) which regulates the release of cytokines like IL-2, IL-3, GM-CSF (Granulocyte colony stimulating factor) and TNF-α [15]. Main clinical effect of cyclosporin-A is probably the inhibition of IL-2 which is produced by T-cells; that which is inhibiting IL-2 that induces proliferation of other T-cells.
An important stage of inflammation after neurol trauma is the activation of microglia and leucocyte infiltration [16]. Microglial cells are known to be the immunity cells of the central neurvous system (CNS). After infiltration of the ischemic tissue by microglia and astrocytes, cytokines (IL-2, IL-3, TNF-α), which are major indicators of the inflammatory response, are released [17]. Cyclosporin-A is defined to be effective in inhibition of TNF-α and cyclooxygenase-2 expression from these microglial cells [18]. From the functional perspective, interestingly cyclosporin-A reduces the formation of NO on the microglial cell line and also probably the cytostatic mechanism is effected as well [19]. Beyond this, following the stimulation of the receptor ligands of the pheriperal benzodiazepines, production of free radicals on different microglial cell lines are blocked by cyclosporin-A [20].
Also cyclosporin-A's effect extends even through astrocytes since it is known that cyclosporin-A prominently reduces astrocytic apoptosis and the release of TNF-α, IL-1 and IL-2 from astrocytes [21,22].
Cyclosporin's effects on acute CNS disorders like cerebral ischemia was first studied on animal experimental models in the early 1990's. This pioneer studies revelead the useful results of cyclosporin on transient ischemic forebrain injury in rats; cyclosporin was reported to reduce the infarct and edema volume [23]. But, it is very rare for cyclosporin to be used in experimental spinal cord injury and acute brain injury models; but beneficial results were reported. For example, the use of cyclosporin after complete thoracal spinal cord cutts in the rats, axonal regeneration is shown to increase and a decrease in inflammatory effects of macrophages and microglial cells was reported [24]. Same group of investigators later on reported a recovery in functional levels with cyclosporin treatment in rats with complete thoracal spinal cord cutts [25]. In studies on rats with traumatic brain injury, which investigate cyclosporin's positive effects on neurons,axonal degeneration models revealed that mitochondrial swelling, deposition of β-amiloid precursor proteins and compaction of the neurfilaments were reduced [26,27]. Cyclosporin-A application in first 6 hours, in throcal cord injury models, was shown to inhibit lipid peroxidation and enhancement in the mobility of the rats exposed to lesion [28]. On the other hand in a more recent study objected these findings, reporting no reduction in tissue loss in groups which were treated with cyclosporin and no enhancement in the motor scores [29]. But later on it has been claimed that this failure of the drug was due to insufficient dosing of the cyclosporin [30]. Most lately a study in 2010, reported that cyclosporin, which is used after transportation of oligodendrocyte precursor cells (OPC) to the traumatized spinal cord, had better histological outcomes and in correlation to this better functional recovery results were achieved by cyclosporin, when compared to the control group; but cyclosporin failed to prevent the transported oligodendrocyte precursor cells to transform into astrocytes [31]. In fluidpercussion injury models cycloporin-A was reported to improve motor and amnestic functions in traumatic brain injury [32]. Reports, that explaining cyclosporin's effects on isolated neurons are very rare. But studies on experimentally lesioned neuroblastoma cells showed that cyclosporin prevents cells from apoptosis, inhibits caspase activation and activates neuronal growth [33,34].
Phase-II clinical researches on traumatic brain injuries are being carried out and those trials are showing the safety of cyclosporin-A. But there is a heterogenicity regarding these trials especially in timing of cyclosporin-A treatment after traumatic beyin injury [35].
There is not any clinical study in the literature about cyclosporin-A treatment in traumatic spinal cord injuries yet. And to our knowledge, no reports of clinical use of cyclosporin-A in acute CNS disorders (such as ischemia) exist. By in vitro and in vivo evaluations of these findings it is accepted that Cyclopsorin-A has direct inhibitor effects on microglial cells, it has neuroprotective and neuroregenerative characteristics and cyclosporin-A has direct effects on neurons and glial cells [36].
As mentioned above there are no experimental studies in the literature, which match identically to our study. And also the number of experimental spinal cord injury studies with cyclopsorin, are very limited. Palladini et al. [25] showed that groups which were treated with cyclosporin-A, had structural and functional recovery, in thoracal cord injuries. Also Diaz- Ruiz et al. [28] declared that in groups treated with cyclopsorin-A after thoracal cord contussion, lipid peroxidation was decreased. But in contrast to these outcomes Rabchevcsky et al. [29] reported that in both groups, treated with cyclosporin-A or not, had similar results in functional and structural recovery parameters.
According to the out-comes of the motor score recovery in both trauma groups were not significant up to day 5; whereas after day 5 in favor of "cord injury+cyclosporin group" there was a significant recovery result (Table 2). Also, when groups were analyzed with binary comparison, (Mann-Whitney U test) functional evaluation (motor score) and also structural evaluation (histologically necrosis-apoptosis) had statistically significant outcomes in favor of "cord injury+CSA" (Table 2).
Conclusion
In our experimental rat spinal cord injury model; we introduce that cyclosporin-A has neuroprotective (Necrosis/Apoptosis data in favor of cyclosporin-A group) and regenerative effects (motor score in favor of cyclosporin-A group) on secondary spinal cord injury, with statistically significant data.
References
- Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord 2003; 41: 369-378.
- Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 2010.
- Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD, Weaver LC. The cellular inflammatory response in human spinal cords after injury. Brain 2006; 129: 3249-3269.
- Hamada Y, Ikata T, Katoh S, Nakauchi K, Niwa M. Involvement of an intercellular adhesion molecule 1-dependent pathway in the pathogenesis of secondary changes after spinal cord injury in rats. J Neurochem 1996; 66: 1525-1531.
- McTigue DM, Tani M, Krivacic K, Chernosky A, Kelner GS. Selective chemokine mRNA accumulation in the rat spinal cord after contusion injury. J Neurosci Res 1998; 53: 368-376.
- Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol 2007; 500: 267-285.
- Bethea JR, Dietrich WD. Targeting the host inflammatory response in traumatic spinal cord injury. Curr Opin Neurol 2002; 15: 355-360.
- Bethea JR, Castro M, Keane RW, Lee TT, Dietrich WD. Traumatic spinal cord injury induces nuclear factor-kappaB activation. J Neurosci 1998; 18: 3251-3260.
- Conti A, Cardali S, Genovese T, Di Paola R, La Rosa G. Role of inflammation in the secondary injury following experimental spinal cord trauma. J Neurosurg Sci 2003; 47: 89.
- Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol 2008; 209: 294-301.
- Taoka Y, Okajima K, Uchiba M, Murakami K, Kushimoto S. Role of neutrophils in spinal cord injury in the rat. Neuroscience 1997; 79: 1177-1182.
- Drummond JC, Moore SS. The influence of dextrose administration on neurologic outcome after temporary spinal cord ischemia in the rabbit. Anesthesiology 1989; 70: 64-70.
- Black P, Markowitz RS, Cooper V, Mechanic A, Kushner H. Models of spinal cord injury: Part 1. Static load technique. Neurosurgery 1986; 19: 752-762.
- Kayaalp S. Rasyonal tedavi yonunden tibbi farmakoloji. Ankara, Hacettepe-Tas. 2002.
- Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol 1997; 15: 707-747.
- Gong C, Hoff JT, Keep RF. Acute inflammatory reaction following experimental intracerebral hemorrhage in rat. Brain Res 2000; 871: 57-65.
- Iadecola C, Forster C, Nogawa S, Clark HB, Ross ME. Cyclooxygenase-2 immunoreactivity in the human brain following cerebral ischemia. Acta Neuropathol 1999; 98: 9-14.
- Choi HB, Khoo C, Ryu JK, Van Breemen E, Kim SU, McLarnon JG. Inhibition of lipopolysaccharide-induced cyclooxygenase-2, tumor necrosis factor-a and [Ca2+] i responses in human microglia by the peripheral benzodiazepine receptor ligand PK11195. J Neurochem 2002; 83: 546-555.
- Lockhart BP, Cressey KC, Lepagnol JM. Suppression of nitric oxide formation by tyrosine kinase inhibitors in murine N9 microglia. Br J Pharmacol 1998; 123: 879-889.
- Jayakumar A, Panickar K, Norenberg M. Effects on free radical generation by ligands of the peripheral benzodiazepine receptor in cultured neural cells. Journal of neurochemistry. 2002; 83: 1226-1234.
- Pyrzynska B, Lis A, Mosieniak G, Kaminska B. Cyclosporin A-sensitive signaling pathway involving calcineurin regulates survival of reactive astrocytes. Neurochem Int 2001; 38: 409-415.
- Herman ZS. Immunophilin ligands decrease release of pro-inflammatory cytokines (il-1b, tnf-a and il-2) in rat astrocyte cultures exposed to simulated ischemia in vitro. Pol J Pharmacol 2004; 56: 129-136.
- Shiga Y, Onodera H, Matsuo Y, Kogure K. Cyclosporin A protects against ischemia-reperfusion injury in the brain. Brain Res 1992; 595: 145-148.
- Teichner A, Morselli E, Buttarelli F, Caronti B, Pontieri F, Venturini G. Treatment with cyclosporine A promotes axonal regeneration in rats submitted to transverse section of the spinal cord. Journal fur Hirnforschung 1992; 34: 343-349.
- Palladini G, Caronti B, Pozzessere G, Teichner A, Buttarelli F, Morselli E. Treatment with cyclosporine A promotes axonal regeneration in rats submitted to transverse section of the spinal cord--II--Recovery of function. Journal fur Hirnforschung. 1995; 37: 145-153.
- Büki A, Okonkwo DO, Povlishock JT. Postinjury cyclosporin A administration limits axonal damage and disconnection in traumatic brain injury. J Neurotrauma 1999; 16: 511-521.
- Okonkwo DO, Povlishock JT. An intrathecal bolus of cyclosporin A before injury preserves mitochondrial integrity and attenuates axonal disruption in traumatic brain injury. J Cereb Blood Flow Metab 1999; 19: 443-451.
- Diaz-Ruiz A, Rios C, Duarte I, Correa D, Guizar-Sahagun G. Cyclosporin-A inhibits lipid peroxidation after spinal cord injury in rats. Neurosci Lett 1999; 266: 61-64.
- Rabchevsky AG, Fugaccia I, Sullivan PG, Scheff SW. Cyclosporin A treatment following spinal cord injury to the rat: behavioral effects and stereological assessment of tissue sparing. J Neurotrauma 2001; 18: 513-522.
- Ibarra A, Hauben E, Butovsky O, Schwartz M. The therapeutic window after spinal cord injury can accommodate T cell-based vaccination and methylprednisolone in rats. Eur J Neurosci 2004; 19: 2984-2990.
- Lü HZ, Wang YX, Zhou JS, Wang FC, Hu JG. Cyclosporin A increases recovery after spinal cord injury but does not improve myelination by oligodendrocyte progenitor cell transplantation. BMC neurosci 2010; 11: 127.
- Alessandri B, Rice AC, Levasseur J, DeFord M, Hamm RJ, Bullock MR. Cyclosporin A improves brain tissue oxygen consumption and learning/memory performance after lateral fluid percussion injury in rats. Journal of neurotrauma. 2002; 19: 829-841.
- Capano M, Virji S, Crompton M. Cyclophilin-A is involved in excitotoxin-induced caspase activation in rat neuronal B50 cells. Biochem J 2002; 363: 29-36.
- Sheehan J, Eischeid A, Saunders R, Pouratian N. Potentiation of neurite outgrowth and reduction of apoptosis by immunosuppressive agents: implications for neuronal injury and transplantation. Neurosurg foc 2006; 20: 1-7.
- Lulic D, Burns J, Bae EC, van Loveren H, Borlongan CV. A review of laboratory and clinical data supporting the safety and efficacy of cyclosporin A in traumatic brain injury. Neurosurgery 2011; 68: 1172-1185.
- Hailer NP. Immunosuppression after traumatic or ischemic CNS damage: it is neuroprotective and illuminates the role of microglial cells. Prog Neurobiol 2008; 84: 211-233.