Research Article - Biomedical Research (2017) Volume 28, Issue 6
The therapeutic effects of BMP-2 and IGF-1 on fracture healing in diabetic rats
Xi Li, Wei Ren, Fengyao Wang, Yaguang Zhu, Hui Wang and Jinsong Liu*Department of Orthopedics, First Affiliated Hospital of Kunming Medical University, PR China
- *Corresponding Author:
- Jinsong Liu
Department of Orthopedics
First Affiliated Hospital of Kunming Medical University
No. 295 Xichang Road, Kunming
Kunming 650031, PR China
Accepted on November 07, 2016
Abstract
Our objective is to explore the effects of BMP-2 and IGF-I in promoting fracture healing in diabetic rats. First 30 rats were made into fracture model with diabetes and randomly divided into 2 experimental groups, the rats in group A´ received insulin growth factor-1 (IGF-1) combined with fibrin glue and the rats in group B´ received bone morphogenetic protein-2 (BMP-2) combined with fibrin glue. The rats without any treatment after model establishment were set as control (group A). At 2, 4 and 6 weeks after the surgery, the X-ray of left lower limb was taken, the bone mineral density (BMD) of local fracture site and callus was detected, the fracture healing was observed, the tissue from the end of fracture was observed by hematoxylin-eosin (H&E) staining, the blood glucose and alkaline phosphatase (ALP) were detected, the osteocalcin (OC) was detected by radioimmunoassay, the local BMP-2, IGF-1 and OC were detected by immunohistochemistry and the collagen type I expression was detected by real time polymerase chain reaction (RT-PCR). All the data were analyzed. In the results, Compared with group A, in group A´ and B´ the ALP and OC were significantly increased, which was highest in the 2nd week, and there was no difference in the 6th week; X-ray results showed that the callus was increased, the BMD values were slightly increased; RT-PCR results showed that the collagen type I in local callus was increased which was most significantly in 2nd week and there was no difference in 6th week. Compared with group B´, the collagen type I in local callus tissue in group A´ was slightly higher which was most significant in 2nd week and there was no difference in 6th week. In conclusion, under the same condition, IGF-I or BMP-2 combined with fibrin glue applying at the end of fracture can significantly improve the fracture healing, and IGF-1 has better effects. Local application of fibrin glue combined with BMP-2 or IGF-I for treating fracture in diabetic rats cannot effectively increase the BMD values in the fracture end and callus.
Keywords
Diabetes, Fracture, BMP-2, IGF-1, Collagen type I, Osteocalcin, Healing.
Introduction
In diabetic patients, the fracturing healing is slow, and the rates of nonunion, delayed healing and pseudarthrosis are high, which has been a problematic issue for doctors [1-3]. To find a more effective, safer and noninvasive way to treat fracture in diabetic patients has always been the hotspot [4]. In our previous study, we showed that during the fracture healing in diabetic rats, the decrease of BMP-2 and IGF-I in the end of fracture is the important factor that causes the slow healing, nonunion and delayed healing. There has been no research about whether local application of BMP-2 and IGF-I can effectively improve the fracture healing in diabetic rats.
BMP-2 and IGF-I are both water-soluble, they can be easily diluted if they are applied locally so that they not able to be effective [5,6]. The recent studies have shown that as an injectable controlled -release vector of cytokines, fibrin glue is an effective way to solve this problem. Fibrin glue can effectively conjugate the ends of fracture, supply cytokines and can be finally degraded [7-9]. The local application of fibrin glue in treating fracture can avoid the injury by surgery, wound infection and nonunion. In one study, fibrin glue was used as injectable vector combined with BMP-2 and bFGF to repair radius defect in dogs and it turned out that the therapeutic efficacy was good [10,11]. In another study, bBMP combined with fibrin glue was effective in treating osteoporosis of spine in sheep [12,13]. However, there has been no research about BMP-2 or IGF-I combined with fibrin glue in treating fracture in diabetic patients.
In our study, we established an animal model and applied BMP-2 or IGF-I combined with fibrin glue in treating fracture healing in diabetic rats. The fracture healing was observed and evaluated to explore the therapeutic effects of BMP-2 and IGF-I in treating fracture in diabetic rats, which further provides an animal experiment basis for clinical treatment of fracture patients with diabetes.
Materials and Methods
Animals
A sum of 60 healthy male SD (Sprague-Dawley) rats aged 3 months were provided by the Experimental Center of Kunming Medical College. The room temperature was maintained at 18-22 and the relative humidity was maintained at 40%-70%.
Instruments and reagents
Fibrin glue was purchased from Beite Biotechnology Co., Ltd, Guangzhou, China, including four parts (biogel, biogel dissolving solution, catalyst and catalyst dissolving solution). Strepozotocin (STZ) was purchased from Sigma-Aldrich Co. LLC., Missouri, USA; OC kit was purchased from Northern Biotechnology Company, Beijing, China; rat BMP-2, IGF-I and OC monoclonal antibodies (rabbit anti rat) and SABC immunohistochemistry kits were purchased from Abcam plc., Cambridge, England; Opticon Real-time PCR amplifier was purchased from MJ Research Inc., Florida, USA.
Grouping and model establishment
30 rats were first made into fracture model with diabetes and randomly divided into 2 experimental groups, the rats in group A´ received insulin growth factor-1 (IGF-1) combined with fibrin glue and the rats in group B´ received bone morphogenetic protein-2 (BMP-2) combined with fibrin glue. The rats without any treatment after model establishment were set as control (group A).
First the diabetic model was established, and then the rats received intraperitoneal injection of STZ (50 mg/kg) in 0.1 mmol/L citric acid-sodium citrate buffer, after 24 hours the blood glucose was detected from the tail, after model establishment the body weight and blood glucose were detected. The criterion for model establishment was randomly non-fasting blood glucose ≥ 13.8 mmol/L. After anesthesia, the upper section of left tibia was exposed and the tibia was cut by scissor at 1 cm from tibial plateau.
IGF-I and BMP-2 were applied according to the body weight (4 ug/kg), and fibrin glue was used as vector (the concentration of both factors were 2 ug/ml). Before surgery, IGF-I or BMP-2 was dissolved in the biogel dissolving solution, and mixed with the powder of biogel to prepare IGF-I or BMP-2/biogel suspension. After that, the powder of catalyst and the catalyst dissolving solution were mixed to prepare catalyst solution. The proportion of two kinds of solutions was 1:1. After fracture model was completed, both solutions were immediately injected into the fracture end and coagulated into gel locally. Then the tibia was fixed by splint.
Detection of blood glucose and ALP in serum
At 2, 4 and 6 weeks after the surgery, 5 SD rats were randomly selected for blood glucose and ALP detection. The blood sample was collected from femoral artery, and the blood ALP was detected by automatic biochemical analyzer (Olmypus Co., Tokyo, Japan).
Serum OC detection by radioimmunoassay (RIA)
After 2, 4 and 6 weeks after model establishment, 5 SD rats were randomly selected. 2 ml blood was collected from femoral artery, centrifuged at 3000 rpm for 15 minutes to collect the supernatant at 4. 125I-BGP and the OC in the standard competitively bind to the OC antibody. The mixture was incubated at 4 for 20 hours to reach the balance, and then the separating reagent was added and incubated at room temperature for 15 minutes. The separating reagent can bind to the OC which binds to antibody to form precipitation, the free OC was centrifuged and discarded. The radiocounting of precipitation was detected and the OC level was calculated.
X-ray and BMD detection
At 2, 4 and 6 weeks after model establishment, 5 SD rats were randomly selected from either group. The rats were sacrificed by intraperitoneal injection of 20 mg/kg 3% pentobarbital sodium. Then the X-ray of the local fracture end was taken and the BMD values were detected.
Hematoxylin-eosin (H & E) staining
After the fracture end was fixed by 4% triformol for 24 hours, decalcified by 5% methanoic acid for 24-48 hours, the slides were embedded and sliced into 5 μm slides. After H&E staining, the proliferation of fracture end and callus and the change of bone trabecula were observed.
Local BMP-2, IGF-I and OC detected by immunohistochemistry
After sacrifice, a part of callus tissue was collected for immunohistochemistry. The tissue was fixed, embedded, sliced, dewaxed and dehydrated. Then the primary antibody and secondary antibody were added and developed for 5 minutes. The brown color represents the location of the antigen. PBS was used as negative control. The expression levels of BMP-2, IGF-1 and OC were evaluated according to the density and amount of positive staining.
Local collagen Type 1 detected by RT-PCR
The local callus tissue was collected and grinded, homogenized and precipitated to extract the RNA. Then the RNAs were transversely transcripted. Real-time PCR: denaturation at 95 for 4 minutes, then 58 for 30 sec, 72 for 40 sec and 76 for 2 sec to detect the florescence, there were 40 cycles in total. Meanwhile, the melting curve between 53-95 was made.
Statistical analysis
SPSS 11.5 was used to analyze the data. All the data were presented as mean ± standard deviation (͞x ± S). One-way ANOVA was used to compare the differences between the groups. P<0.05 was considered as statistically significant.
Results
General characteristics
In group A´ and B´, after the first injection of STZ all the animals had different degrees of spirit atrophy, slow reaction, emaciation, decreased body weight, increased diet and diuresis, which were aggravated with the time. Local application of BMP-2 or IGF-1 could not improve the systematic response of rats.
In the rats after local application of BMP-2 or IGF-I, the diet of rats gradually increased after 1 week, the mortality was decreased, the mental state was improved and the activity was increased. After 4 weeks, the general status was worse again. The systematic status was similar to the rats in group A.
Detection of blood glucose and ALP
Local application of BMP-2 or IGF-I did not significantly affect the blood glucose in diabetic rats. As shown in Table 1.
| Group A´ | Group B´ | Group A | F value | P value | |
|---|---|---|---|---|---|
| 2nd week | 17.42 ± 0.69 | 16.78 ± 1.09 | 16.33 ± 1.93 | 1.095 | 0.366 |
| 4th week | 16.55 ± 1.07 | 17.07 ± 0.83 | 16.43 ± 1.20 | 0.530 | 0.601 |
| 6th week | 16.14 ± 2.56 | 16.15 ± 1.98 | 16.13 ± 1.33 | 0.140 | 0.871 |
Table 1. The dynamic change of blood glucose in different groups (͞x ± s, mmol/L, n=5).
The ALP levels in group A´ and B´ were not statistically different at 2nd, 4th and 6th week. The ALP levels in group A´ and B´ were significantly higher than group A, which were most significantly different in the 2nd and 4th week (P<0.01), and different in the 6th week (P<0.05). As shown in Table 2.
| Group A´ | Group B´ | Group A | F value | P value | |
|---|---|---|---|---|---|
| 2nd week | 417.0 ± 12.7 | 406.2 ± 21.6 | 236.2 ± 19 | 154.491 | 0.000 |
| 4th week | 291.6 ± 13.5 | 275.7 ± 10.6 | 209.5 ± 13.6 | 59.394 | 0.000 |
| 6th week | 230.7 ± 19.2 | 212.1 ± 11.6 | 168.2 ± 11.1 | 24.685 | 0.000 |
Table 2. The dynamic change of blood ALP in different groups ( ͞x ± s, IU/L, n=5).
Detection of serum OC
The trends of serum OC and ALP were consistent. The OC levels were not statistically different between group A´ and B´ in the 2nd, 4th and 6th week. The OC levels were significantly higher in group A´ and group B´ than group A, which were most significantly different in 2nd and 4th week (P<0.01), and different in the 6th week (P<0.05). As shown in Table 3.
| Group A´ | Group B´ | Group A | F value | P value | |
|---|---|---|---|---|---|
| 2nd week | 2.471 ± 0.164 | 2.346 ± 0.185 | 1.906 ± 0.098 | 18.605 | 0.000 |
| 4th week | 2.114 ± 0.102 | 2.054 ± 0.133 | 1.788 ± 0.109 | 11.161 | 0.002 |
| 6th week | 1.826 ± 0.105 | 1.797 ± 0.062 | 1.428 ± 0.079 | 34.856 | 0.000 |
Table 3. The dynamic change of OC in different groups ( ͞x ± s, μg/L, n=5)
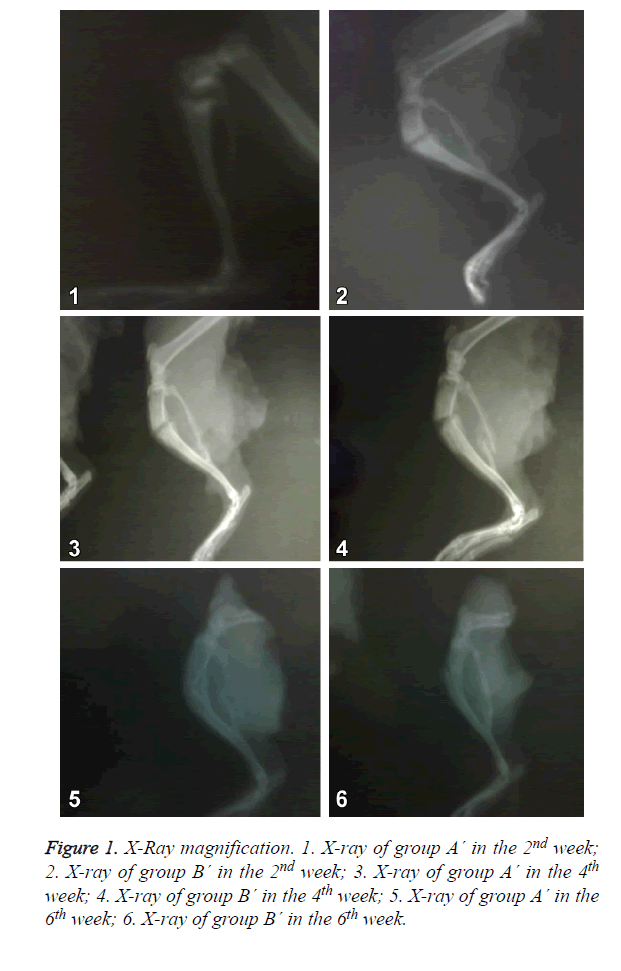
X-Ray magnification
Group A´: in the X-ray of the 2nd week, there was callus formation and callus shadow. In the X-ray of the 4th and 6th week, the transmittance of callus shadow was further decreased and there was fracture healing.
The X-rays in group A´ and group B´ were similar, and both were better than group A. As shown in Figure 1.
The BMD values in local fracture end and callus
The BMD values in group A´ and group B´ were similar to group A in the 2nd, 4th and 6th week, which were not statistically different. The values in the 3 groups were all decreased with the time. As shown in Table 4.
| Group A´ | Group B´ | Group A | F value | P value | |
|---|---|---|---|---|---|
| 2nd week | 0.195 ± 0.009 | 0.191 ± 0.011 | 0.187 ± 0.015 | 0.283 | 0.758 |
| 4th week | 0.171 ± 0.007 | 0.169 ± 0.010 | 0.163 ± 0.009 | 1.412 | 0.281 |
| 6th week | 0.154 ± 0.009 | 0.155 ± 0.010 | 0.153 ± 0.012 | 0.046 | 0.955 |
Table 4. BMD values in local fracture end and callus (g/cm2) ( ͞x ± s, n=5).
General observation
Group A´: In the 2nd week, there was fibrous tissue in the fracture end, however the fracture end was still clear. In the 4th week, there was bone tissue in the fracture end wrapped with fibrous tissue, the fracture end was clear. In the 6th week, the fracture end was connected by bone tissue wrapped with fibrous tissue, and fracture end was hard to distinguish.
Group B´: In the 2nd week, there was fibrous tissue in the fracture end, however, the fracture end was still clear. In the 4th week, there was bone tissue in the fracture end wrapped with fibrous tissue, the fracture end was clear. In the 6th week, the fracture end was connected by bone tissue wrapped with fibrous tissue, and fracture end was hard to distinguish.
Both the groups were better than group A.
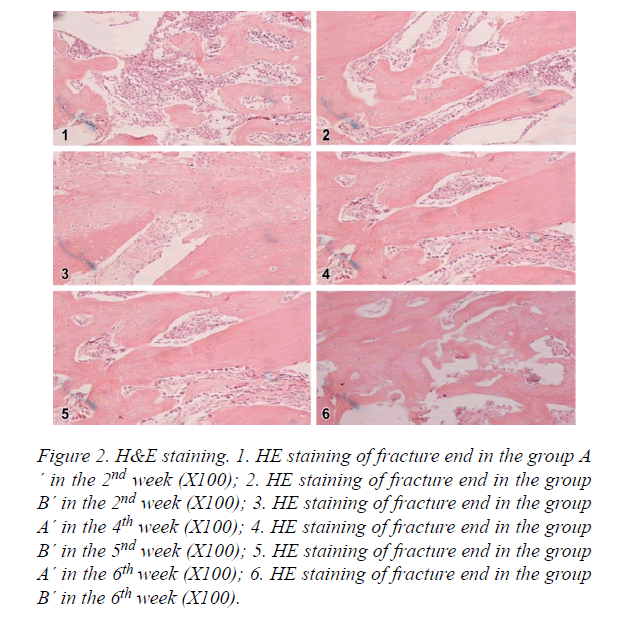
H&E staining
Group A´: As shown in Figure 2, in the 2nd week, there were a large amount of bone trabecula, osteoblasts and chondrocytes. In the 4th week, there was bone trabecula connection in intramembranous ossification zone, osteoblasts transferred to osteocytes, part of bone trabecula merged together, and cartilage callus was substituted by new bone trabecula. In the 6th week, bone trabecula started to transfer to lamellar bone, there was a few of fibrous callus and a lot of osteoclasts.
Figure 2. H&E staining. 1. HE staining of fracture end in the group A ´ in the 2nd week (X100); 2. HE staining of fracture end in the group B´ in the 2nd week (X100); 3. HE staining of fracture end in the group A´ in the 4th week (X100); 4. HE staining of fracture end in the group B´ in the 5nd week (X100); 5. HE staining of fracture end in the group A´ in the 6th week (X100); 6. HE staining of fracture end in the group B´ in the 6th week (X100).
Group B´: As shown in Figure 2, in the 2nd week, there were some bone trabecula, osteoblasts and chondrocytes. In the 4th week, there was bone trabecula connection in intramembranous ossification zone, osteoblasts transferred to osteocytes, part of bone trabecula merged together, cartilage callus was substituted by new bone trabecula. In the 6th week, bone trabecula started to transfer to lamellar bone, there was a few of fibrous callus and a lot of osteoclasts.
The amount of osteoblasts and callus density in group A´ and B´ were higher than group A. However, there was no significant difference between group A´ and group B´. Group A´ was slightly better than group B´.
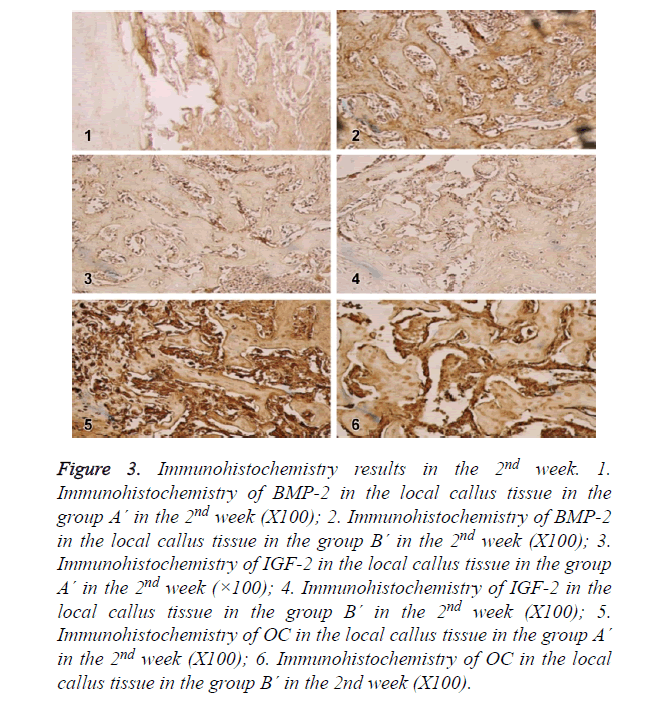
Immunohistochemistry
In the 2nd week, BMP-2, IGF-I and OC in group A´ and group B´ reached the peak, showing high expression in fibroblasts, osteoblasts, mature chondrocytes and bone matrix. The expression levels in both groups were higher than group A, which were most significant in the 2nd week. As shown in Figure 3.
Figure 3. Immunohistochemistry results in the 2nd week. 1. Immunohistochemistry of BMP-2 in the local callus tissue in the group A´ in the 2nd week (X100); 2. Immunohistochemistry of BMP-2 in the local callus tissue in the group B´ in the 2nd week (X100); 3. Immunohistochemistry of IGF-2 in the local callus tissue in the group A´ in the 2nd week (×100); 4. Immunohistochemistry of IGF-2 in the local callus tissue in the group B´ in the 2nd week (X100); 5. Immunohistochemistry of OC in the local callus tissue in the group A´ in the 2nd week (X100); 6. Immunohistochemistry of OC in the local callus tissue in the group B´ in the 2nd week (X100).
In the 4th week, BMP-2, IGF-I and OC were positive in the same kinds of cells as group A´ and B´ with decreased expression levels. As the degradation of fibrin glue, BMP-2 and IGF-I were both absorbed, which were both decreased in group A´ and group B´, and there was no difference compared with group A. As shown in Figure 4.
In the 6th week, the cells which expressed BMP-2, IGF-I and OC were decreased in group A´, B´ and A. They were expressed in a few of osteoblasts and osteoclasts, and there was no statistical difference.
Collagen type I detected by RT-PCR
Type I collagen mRNA in group A´ and group B´ were significantly higher than group A, which were most significant in the 2nd and 4th week (P<0.01) and different in the 6th week (P<0.05). There was no difference between group A´ and group B´ in the 4th and 6th week (P>0.05). It was higher in group A´ than group B´ in the 2nd week, there was statistical difference (P<0.05). As shown in Table 5.
| Group A´ | Group B´ | Group A | F value | P value | |
|---|---|---|---|---|---|
| 2nd week | 1.432 ± 0.064 | 1.360 ± 0.066 | 1.146 ± 0.045 | 31.367 | 0.0000 |
| 4th week | 1.246 ± 0.071 | 1.207±0.100 | 1.046 ± 0.027 | 15.337 | 0.0000 |
| 6th week | 1.078 ± 0.024 | 1.057 ± 0.040 | 0.728 ± 0.027 | 165.019 | 0.0000 |
Table 5. Type I collagen expression levels in local callus tissue in different groups ( ͞x ± s, n=5).
Discussion
There are many factors that cause the slow fracture healing, nonunion and delayed healing in diabetic rats with fracture [14,15]. Hypoinsulinemia and hyperglycemia (insulin resistance for type 2 diabetes) decrease the secretion of multiple factors that promote fracture healing after trauma, and further decrease the proliferation and delay the maturity of osteoblasts. Then the collagen type I is decreased, bone matrix is decreased, the callus is formed, the reconstruction is slower, and the loss of calcium and phosphorus causes slow fracture healing, nonunion or delayed healing [16]. There are many ways to treat the fracture healing disorder. At present, insulin is most commonly applied and the blood glucose was dynamically monitored. However, because there is insulin resistance in type 2 diabetes patients, the efficacy of insulin is poor [17-19]. Some studies have applied skeletal growth factors systematically or locally to improve the fracture healing in diabetic rats with fracture, however, because most of the skeletal growth factors are water soluble, the local application of them can be easily diluted and degraded [20,21].
In our previous studies, we found that secretion of BMP-2 and IGF-1 in diabetic rats with fracture was decreased. BMP-2 and IGF-I can quickly diffuse in the body and can be easily degraded by protease, thus it cannot keep its bone induction effect in an effective time. Thus, choosing an appropriate biomaterial as the controlled-release vector of BMP-2 and IGFI is also important in bone tissue engineering research. In diabetic patients, there is often would infection and the healing is difficult. Minimally invasive treatment can avoid the wound infection caused by the surgery, and the healing disorder is very critical [22,23]. Injectable vector combined with some cytokines applied locally can promote the fracture healing, which is significant in the treatment of diabetic patients with fracture.
Fibrin glue is bioprotein product, which is applied for hemostasis of wound. In the recent 20 years, it is gradually applied for promoting wound healing, seal tissue defect and prevent tissue adhesion, especially as the controlled-release vector of some drugs [24,25]. Bergel for the first time reported the hemostasis function of fibrin glue powder [26]. Nowadays, the fibrin glue is mostly extracted from animals, it can form stable insoluble fibrous protein polymer to develop hemostasis, sealing and adhesion effects.
After mixing, the gel can form a net rack structure like a sponge, the mesh can be a space for drug storage. As the gel is absorbed, the drug is slowly released. Because fibrin glue is relatively stable in the body, it is gradually degraded and absorbed between 3rd and 4th week. Thus, it can temporarily preserve and slowly release the drug, and increase the detention time, increase the local drug concentration and decrease the drug that enters the blood circulation [27-30]. The critical healing time for fracture is the 1st to 4th week. In our previous study, we showed that BMP-2, IGF-I, OC and collagen type I in diabetic rats were lower than the normal rats, which were most significant in the 1st week to the 4th week.
Compared with group A, the BMD values in fracture end and callus tissue in group A´ and B´ were increased after 1-3 weeks, but there was no significant difference and there was difference after 4 weeks. Considering that there is local callus mineralization disorder in the late stage fracture healing, the activity of osteoclasts is increased, thus there is fracture healing disorder; fibrin glue combined with BMP-2 and IGF-I are mainly for the early stage osteogenesis, the time of fibrin glue degradation is usually less than 3-4 weeks, thus the local application of BMP-2 and IGF-I doesn’t significantly affect the disorder of local bone and callus mineralization disorder. The critical factors that determine the BMD value of local bone and callus is the mineral content level in the local tissue. Local application of BMP-2 and IGF-I combined with fibrin glue can for treating diabetic rats with fracture cannot stop the loss of calcium and phosphorus or improve the precipitation in local callus tissue; it cannot stop the decrease of BMD values in local bone and callus, which doesn’t significantly affect the mineralization of local callus. Thus, we consider that it has little intervention on late stage healing of fracture.
Immunohistochemistry results showed that in group A´ and group B´, BMP-2 and IGF-I expression reached the peak in the 2nd week, which were significantly different from group A, and the expression was gradually decreased since then, which was only expressed in some osteoclasts in the 6th week. It suggests that BMP-2 and IGF-I has positive feedback effect. The significant decrease in the 4th week indicates that the degradation time of fibrin glue is usually less than 4 weeks. OC in group A´ and group B´ reached the peak in the 2nd week, which was most significant in the 2nd to 4th week and not different in the 6th week. It indicates that local application of BMP-2 or IGF-I can significantly up-regulate the local expression of OC in diabetic rats with fracture. H&E staining showed that the proliferation of osteoblasts was significantly increased after application of BMP-2 and IGF-I. The data in group A´ were higher than group B´ at the 3 time points, however, it was only statistically significant in the 2nd week. The trend of OC and ALP expression in group A´ and B´ was the same to the local OC expression, which further reflect the enhancement of osteogenesis.
PCR results showed that collagen type I mRNA in group A´ and B´ reached the peak in the 2nd week, there was significant different from group A in the 2nd and 4th week and there was no difference in the 6th week, which was in accordance with the peak of OC. Local application of BMP-2 and IGF-I could both promote the expression of collagen type I and promote the fracturing healing in diabetic rats. The data in group A´ were higher than group at the 3 time points, however, it was only statistically significant in the 2nd week.
Although local application of BMP-2 or IGF-I combined with fibrin glue cannot effectively prevent the decrease of local BMD, it has obvious osteogenesis effect in the first 4 weeks which are the critical stage of fracture healing. It can effectively promote the fracture healing in diabetic rats.
In conclusion, under the same condition, IGF-I or BMP-2 combined with fibrin glue applying at the fracture end can significantly improve the fracture healing, and IGF-1 has better effects. Local application of fibrin glue combined with BMP-2 or IGF-I for treating fracture in diabetic rats cannot effectively increase the BMD values in the end of fracture and callus.
Acknowledgement
This work was supported by the Doctoral Fund of First Affiliated Hospital of Kunming Medical University (No. 201313503).
References
- Weber DR, Haynes K, Leonard MB, Willi SM, Denburg MR. Response to Comment on Weber et al. Type 1 Diabetes is Associated With an Increased Risk of Fracture Across the Life Span: A Population-Based Cohort Study Using The Health Improvement Network (THIN). Diabetes Care 2015; 38: 1913-1920.
- Shah VN, Shah CS, Snell-Bergeon JK. Type 1 diabetes and risk of fracture: meta-analysis and review of the literature. Diabet Med 2015; 32: 1134-1142.
- Watts NB, Bilezikian JP, Usiskin K, Edwards R, Desai M. Effects of Canagliflozin on Fracture Risk in Patients With Type 2 Diabetes Mellitus. J Clin Endocrinol Metab 2016; 101: 157-166.
- Campbell GM, Tiwari S, Picke AK, Hofbauer C, Rauner M, Morlock MM, Hofbauer LC, Glüer CC. Effects of insulin therapy on porosity, non-enzymatic glycation and mechanical competence in the bone of rats with type 2 diabetes mellitus. Bone 2016; 91: 186-193.
- Cheng TL, Schindeler A, Little DG. BMP-2 delivered via sucrose acetate isobutyrate (SAIB) improves bone repair in a rat open fracture model. J Orthop Res 2016; 34: 1168-1176.
- Lundin H, Sääf M, Strender LE, Nyren S, Johansson SE, Salminen H. High Serum Insulin-Like Growth Factor-Binding Protein 1 (IGFBP-1) is Associated with High Fracture Risk Independent of Insulin-Like Growth Factor 1 (IGF-I). Calcif Tissue Int 2016; 99: 333-339.
- Li Y, Li R, Hu J, Song D, Jiang X, Zhu S. Recombinant human bone morphogenetic protein-2 suspended in fibrin glue enhances bone formation during distraction osteogenesis in rabbits. Arch Med Sci 2016; 12, 494-501.
- Stoikes N, Sharpe J, Tasneem H, Roan E, Paulus E, Powell B, Webb D, Handorf C, Eckstein E, Fabian T, Voeller G. Biomechanical evaluation of fixation properties of fibrin glue for ventral incisional hernia repair. Hernia 2015; 19, 161-166.
- Sakaguchi Y, Tsuji Y, Ono S, Saito I, Kataoka Y, Takahashi Y, Nakayama C, Shichijo S, Matsuda R, Minatsuki C, Asada-Hirayama I, Niimi K, Kodashima S, Yamamichi N, Fujishiro M, Koike K. Polyglycolic acid sheets with fibrin glue can prevent esophageal stricture after endoscopic submucosal dissection. Endoscopy 2015; 47, 336-340.
- Bhardwaj N, Devi D, Mandal BB. Tissue-engineered cartilage: the crossroads of biomaterials, cells and stimulating factors. Macromol Biosci 2015; 15: 153-182.
- Lv J, Xiu P, Tan J, Jia Z, Cai H, Liu Z. Enhanced angiogenesis and osteogenesis in critical bone defects by the controlled release of BMP-2 and VEGF: implantation of electron beam melting-fabricated porous Ti6Al4V scaffolds incorporating growth factor-doped fibrin glue. Biomed Mater 2015; 10: 035013.
- Schmitt C, Lutz R, Doering H, Lell M, Ratky J. Bio-Oss® blocks combined with BMP-2 and VEGF for the regeneration of bony defects and vertical augmentation. Clin Oral Implants Res 2013; 24: 450-460.
- van der Stok J, Koolen MK, de Maat MP, Yavari SA, Alblas J, Patka P, Verhaar JA, van Lieshout EM, Zadpoor AA, Weinans H, Jahr H. Full regeneration of segmental bone defects using porous titanium implants loaded with BMP-2 containing fibrin gels. Eur Cell Mater 2015; 2015: 141-154.
- Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol 2012; 8: 133-143.
- Metsemakers WJ, Handojo K, Reynders P, Sermon A, Vanderschot P, Nijs S. Individual risk factors for deep infection and compromised fracture healing after intramedullary nailing of tibial shaft fractures: a single centre experience of 480 patients. Injury 2015; 46: 740-745.
- Jiao H, Xiao E, Graves DT. Diabetes and Its Effect on Bone and Fracture Healing. Curr Osteoporos Rep 2015; 13: 327-335.
- Looker AC, Eberhardt MS, Saydah SH. Diabetes and fracture risk in older U.S. adults. Bone 2016; 82: 9-15.
- Srikanthan P, Crandall CJ, Miller-Martinez D, Seeman TE, Greendale GA, Binkley N, Karlamangla AS. Insulin resistance and bone strength: findings from the study of midlife in the United States. J Bone Miner Res 2014; 29: 796-803.
- de Paula FJ, de Araújo IM, Carvalho AL, Elias J Jr, Salmon CE. The Relationship of Fat Distribution and Insulin Resistance with Lumbar Spine Bone Mass in Women. PLoS One 2015; 10: e0129764.
- Poniatowski L A, Wojdasiewicz P, Gasik R, Szukiewicz D. Transforming growth factor beta family: insight into the role of growth factors in regulation of fracture healing biology and potential clinical applications. Mediators Inflamm 2015; 2015, 137823.
- Deng Z, Xu B, Jin J, Zhao J, Xu H. Strategies for Management of Spinal Metastases: A Comprehensive Review. Cancer Transl Med 2015; 3, 94-100.
- Mahajan S, Saoji A A, Agrawal A. Utility of Fine Needle Aspiration Cytology in Diagnosing Bone Tumors. Cancer Trans Med 2015; 5, 166-169.
- Peters EJ, Lipsky BA, Aragón-Sánchez J, Boyko EJ, Diggle M, Embil JM, Kono S, Lavery LA, Senneville E, Urbancic-Rovan V, Van Asten SA, Jeffcoate WJ. International Working Group on the Diabetic Foot Interventions in the management of infection in the foot in diabetes: a systematic review. Diabetes Metab Res Rev 2016; 32, 145-153.
- Carandina S, Tabbara M, Bossi M, Valenti A, Polliand C, Genser L, Barrat C. Staple line reinforcement during laparoscopic sleeve gastrectomy: absorbable monofilament, barbed suture, fibrin glue, or nothing? Results of a prospective randomized study. J Gastrointest Surg 2016; 20, 361-366.
- Kang N, Song SH, Kyung H, Oh SH. Medpor Implant Fixation Using Fibrin Glue in the Treatment of Medial Orbital Wall Fracture. J Craniofac Surg 2015; 26: 1361-1364.
- Mushi E, Kinshuck A, Svecova N, Schache A, Jones TM, Tandon S, Lancaster J. The use of Tisseel™ fibrin sealant in selective neck dissection–a retrospective study in a tertiary Head and Neck Surgery centre. Clin Otolaryngol 2015; 40, 93-97.
- Jackson MR. Fibrin sealants in surgical practice: An overview. Am J Surg 2001; 182: 1S-7S.
- Roura S, Gálvez-Montón C, Bayes-Genis A. Fibrin, the preferred scaffold for cell transplantation after myocardial infarction? An old molecule with a new life. J Tissue Eng Regen Med 2016.
- Shi Z, Fan X, Zhai S, Zhong X, Huang D. Fibrin glue versus staple for mesh fixation in laparoscopic transabdominal preperitoneal repair of inguinal hernia: a meta-analysis and systematic review. Surg Endosc 2016: 1-11.
- Smith CM, Jones A, Dass D, Murthi G, Lindley R. Early experience of the use of fibrin sealant in the management of children with pilonidal sinus disease. J Pediatr Surg 2015; 50: 320-322.