Research Article - Biomedical Research (2017) Volume 28, Issue 4
The study on the inhibitory effect of rPI-T1 on kallikrein
Hongying Wang1, Mei Xu1*, Na Li1, Yan Xue1, Dong Sun1, Weiqing Di1, Zhongfu Ding1, Liquan Yang21Liaoning Grand Nuokang Biopharmaceutical CO., LTD, Shenyang of Liaoning province, China
2College of Pharmacy, Jilin University, Changchun of Jilin Province, China
- *Corresponding Author:
- Mei Xu
Liaoning Grand Nuokang Biopharmaceutical CO., LTD
Liaoning province, China
Accepted on August 29, 2016
Abstract
To comparatively study the effects of rPI-T1 with aprotinin and tranexamic acid, antifibrinolytic hemostatics currently used in clinical settings, by comparatively analyzing their inhibitory activities on plasmin, trypsin and kallikrein. Spectrophotometry was used, that was, OD405 was decreased by inhibiting protease hydrolysis of corresponding chromogenic substrates with inhibitors to measure corresponding activities and Ki values. Under the same measurement conditions, kallikrein inhibitory activity of rPI-T1 was approximately 1% of aprotinin. Tranexamic acid lysine analogues, on the other hand, had millimolar inhibitory concentrations because of different mechanism of action, i.e. noncompetitive inhibition. Due to the weak inhibitory effect of rPI-T1 on kallikrein, the potential side effects resulting from inhibiting kallikrein can be avoided. Characterized by high inhibitory concentration and poor specificity, tranexamic acid is prone to cause severe side effects.
Keywords
Kunitz inhibitor, Plasmin inhibitor, Antifibrinolytic hemostatic, Recombinant protein
Introduction
Serine protease inhibitors are widely present in animals, plants and microorganisms, which can precisely regulate protease activity. Bovine pancreatic trypsin inhibitor (BPTI), as a firstly discovered Kunitz-type protease inhibitor molecule, is a classic representative of its protein family. Aprotinin is a protein with the same sequence as BPTI extracted from bovine lungs, which is a main ingredient of hemostatic aprotinin injection. The injection can be used for treatment and prevention of acute hemorrhage and disseminated intravascular coagulation caused by fibrinolysis [1,2]. However, according to the data reported by the National Center for Adverse Drug Reaction Monitoring in December 2007, aprotinin injection can cause allergy, shock, palpitation, chest tightness, dyspnea, fever and vomiting, which was thus delisted. Textilinin-1 is an active protein extracted from Australian brown snake, Pseudonaja textilis. It is a Kunitz-type serine protease inhibitor containing 59 amino acids and 3 pairs of disulfide bonds, which has a molecular weight of about 6.7 kDa [3,4]. Enzyme kinetic studies have shown that textilinin-1 has strong inhibitory effects on plasmin and trypsin; weak inhibitory effects on kallikrein; and no inhibitory effect on thrombin, factor Xa, urokinase or t-PA [5,6]. Aprotinin has strong inhibitory effects on plasmin, kallikrein and trypsin; and varying inhibitory effects on factor Xa, urokinase and t-PA. Furthermore, plasmin inhibited by textilinin-1 is quickly reversed, which is easier to control than aprotinin and can reduce the risk of thrombus caused by excessive plasmin suppression [7]. Recombinant textilinin-1 (rPI-T1) used in this study is the recombinant protein of textilinin-1 obtained using Pichia pastoris as expression system [8]. Under the same measurement conditions, inhibitory activities of rPI-T1, aprotinin and tranexamic acid on plasmin, trypsin and kallikrein were compared.
Materials and Methods
Materials and instruments
rPI-T1; colorless clear liquid; HPLC (purity 97.0%); protein (concentration 28 mg/ml, provided by Liaoning Nuokang Biopharma LLC). EP tubes, centrifuge tubes and pipette tips were imported products of Haimen Company. 759115 Semimicro cuvettes (Brand). S-2765, S-2251 and S-2302 were purchased from Chromogenix. Meo-suc-AAPV-PNA, plasmin, trypsin and trans-4-(aminomethyl) cyclohexanecarboxylic acid were purchased from Sigma. Kallikrein human plasma and elastase human neutrophil were purchased from Merck. U3900 UV-Vis spectrometer (Hitachi).
Determination of inhibitor constants
Solutions were prepared as follows: 75 μg of Trypsin was dissolved in 750 μl of 2 mM HCl, subpackaged and stored at -20°C. S2765 was dissolved in 50 mM Tris-HCl (pH 7.4) buffer, prepared into 3 mM stock solution and stored at 4°C. Specifically, 8.5 ml of 50 mM Tris-HCl (pH 7.4) buffer was added to S2765-containing reagent bottle, 10 μl of which was then taken and mixed well with 990 μl of 50 mM Tris-HCl (pH 7.4) and measured for OD=0.4494 at 316 nm. S2765 concentration was calculated to be 3.538 mM according to the extinction coefficient ε=12700 L/cm/mol provided in the instructions, which was further diluted to 3 mM. In accordance with the instructions, aprotinin (A6103, sigma) was dissolved in 150 mM NaCl, prepared into 2 mM stock solution and cryopreserved at -20°C. That was, 25 mg of aprotinin was dissolved in 1.9 ml of 150 mM NaCl to a concentration of 2.02 mM, which was then further diluted to 2 mM. Tranexamic acid was prepared each time right before use, which was dissolved in 50 mM Tris-HCl (pH7.4) buffer to a concentration of 200 mM. That was, 15.72 mg of tranexamic acid was dissolved in 0.5 ml of 50 mM Tris-HCl (pH 7.4) buffer. Appropriate amounts of enzymes were taken and incubated with different concentrations of inhibitors at 37°C for 30 min. Then, a different concentration of substrates was added, and A405 time curves were measured at 37°C with a UV-Vis spectrophotometer. Afterwards, curves were plotted with reciprocal of enzymatic reaction velocity given by instrument at a single substrate concentration versus inhibitor concentration. Abscissas of the intersections of fitted lines obtained at different substrate concentrations were precisely Ki.
Inhibition linear range
Appropriate amounts of enzymes were taken and incubated with different concentrations of inhibitors at 37°C for 30 min. Then, a certain concentration of substrates were added, and A405 time curves were measured at 37°C with a UV-Vis spectrophotometer. Afterwards, curves were plotted with enzymatic reaction velocity given by instrument versus inhibitor concentration. Inhibition linear range was determined based on the fitting results.
Results
Plasmin experiment
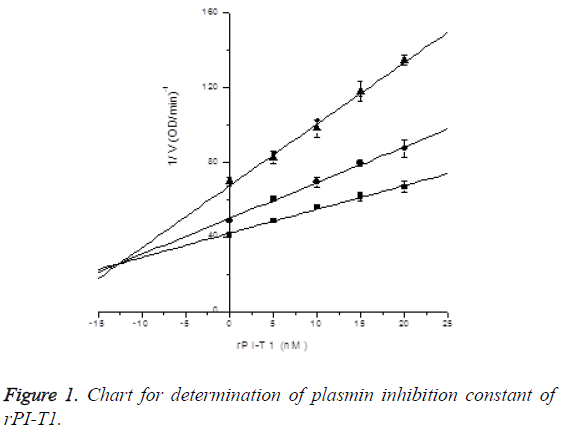
Plasmin inhibition constant of rPI-T1: Final concentration of plasmin was 2.5 μg/ml. In the presence of S-2251 with final concentrations of 0.45 mM, 0.3 mM and 0.15 mM, the inhibitory effects of rPI-T1 with final concentrations of 0, 5, 10, 15 and 20 nM on plasmin activity were measured, and Ki was determined by Dixon plotting. Plasmin inhibition constant of rPI-T1 was determined to be Ki=12.60 ± 1.54 nM; see Figure 1.
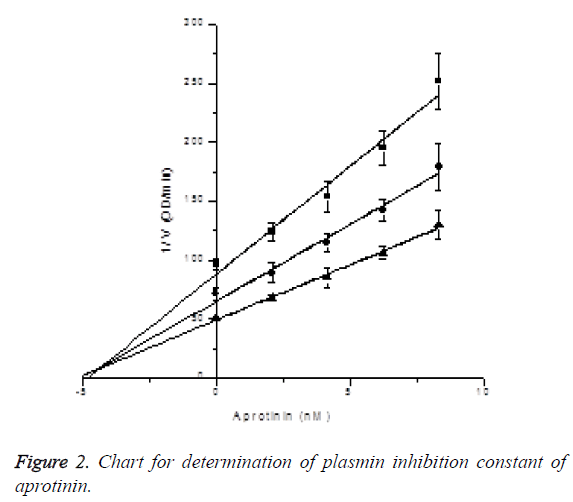
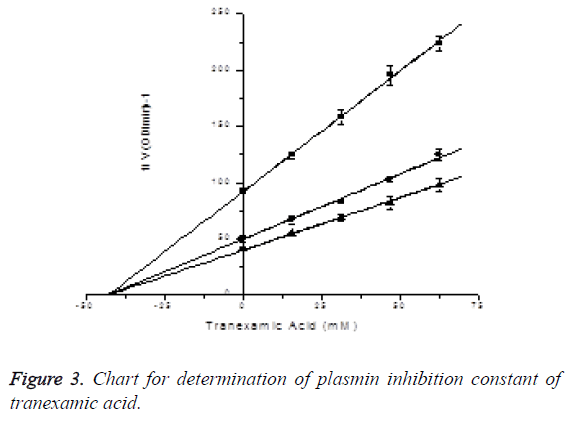
Plasmin inhibition constant of tranexamic acid: In the presence of S-2251 with final concentrations of 450 μM, 300 μM and 150 μM, the inhibitory effects of tranexamic acid with final concentrations of 0, 15.625, 31.25, 46.875 and 62.5 mM on plasmin activity were measured (reacting 0, 150, 300, 450 and 600 μl of tranexamic acid in 960 μl activity measuring system), and Ki was determined by Dixon plotting (Figure 2). Plasmin final concentration was 2.5 μg/ml, while reaction system was 960 μl. Through plotting, the plasmin inhibition constant of tranexamic acid was determined to be Ki=2.73 ± 3.23nM. Since the three lines intersected at X axis, the plasmin inhibitory effect of tranexamic acid was identified to be noncompetitive inhibition. The results are shown in Figure 3.
Trypsin experiment
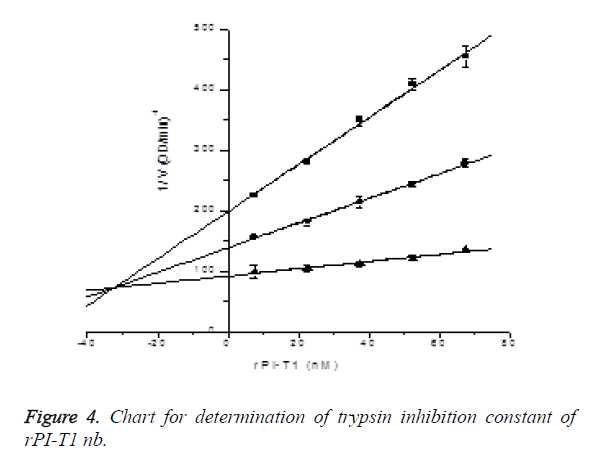
Determination of trypsin inhibition constant of rPI-T1: In the presence of S-2765 with final concentrations of 0.03 mM, 0.06 mM and 0.18 mM, the inhibitory effects of rPI-T1 with final concentrations of 7.5, 22.5, 37.5, 52.5 and 67.5 nM on trypsin activity were measured (reacting 2.4, 7.2, 12, 16.8 and 21.6 μl of 3 μM rPI-T1 in 960 μl activity measuring system), and Ki was determined by Dixon plotting. Ki=32.26242 ± 2.73167 nM, R2=0.99903; the results are shown in Figure 4.
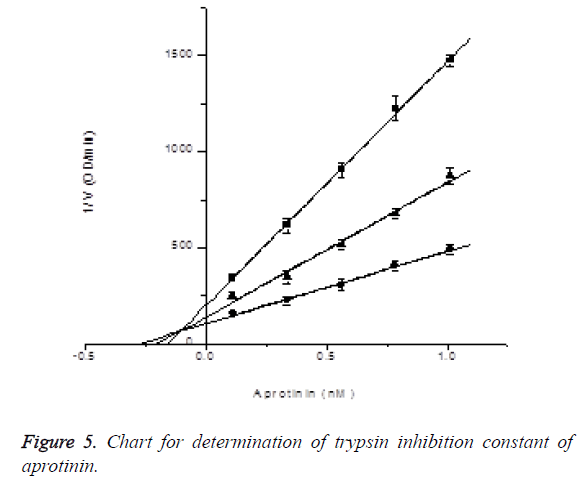
Determination of trypsin inhibition constant of aprotinin: In the presence of S-2765 with final concentrations of 0.03 mM, 0.06 mM and 0.18 mM, the inhibitory effects of aprotinin with final concentrations of 0.1125, 0.3375, 0.5625, 0.7875 and 1.0125 nM on trypsin activity were measured (reacting 2.4, 7.2, 12, 16.8 and 21.6 μl of 45 nM aprotinin in 960 μl activity measuring system), and Ki was determined by Dixon plotting. Trypsin final dosage was 0.025 μg, while reaction system was 960 μl. Ki=0.10604 ± 0.03332 nM, R2=0.99796; the results are shown in Figure 5.
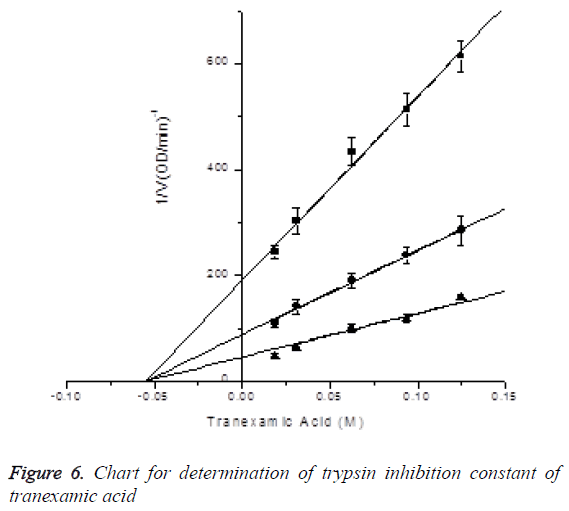
Determination of trypsin inhibition constant of tranexamic acid: In the presence of S-2765 with final concentrations of 0.03 mM, 0.06 mM and 0.18 mM, the inhibitory effects of tranexamic acid with final concentrations of 0.01875, 0.03125, 0.0625, 0.09375 and 0.125 M on trypsin activity were measured (reacting 90, 150, 300, 450 and 600 μl of 0.2 M tranexamic acid in 960 μl activity measuring system), and Ki was determined by Dixon plotting. Trypsin final dosage was 0.025 μg, while reaction system was 960 μl. Ki=0.05493 ± 0.00422 nM, R2=0.99642; the results are shown in Figure 6.
Kallikrein experiment
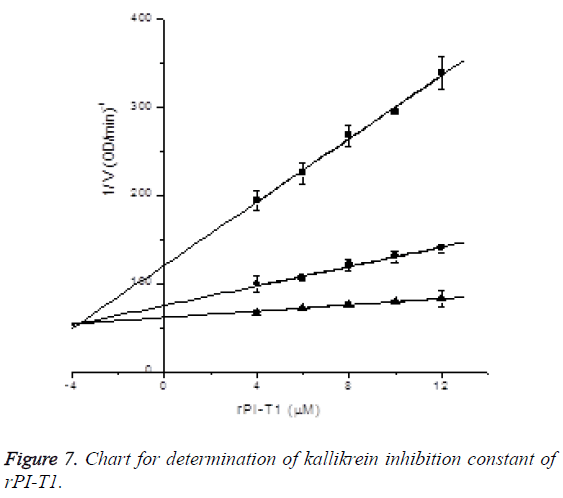
Determination of kallikrein inhibition constant of rPI-T1: In the presence of S-2302 with final concentrations of 0.1 mM, 0.2 mM and 0.4 mM, the inhibitory effects of rPI-T1 with final concentrations of 4, 6, 8, 10 and 12 μM on kallikrein activity were measured (reacting 16, 24, 32, 40 and 48 μl of 240 μM rPI-T1 in 960 μl activity measuring system), and Ki was determined by Dixon plotting. Kallikrein final concentration was 0.175781 μg/ml (after 32-fold diluting 0.54 μg/μl kallikrein into 50 mM Tris-HCl pH 7.4, 10 μl was taken and subjected to activity measurement in the 960 μl system). Through plotting, kallikrein inhibition constant of rPI-T1 was determined to be Ki=3.65912 ± 0.46831 uM, R2=0.99922; the results are shown in Figure 7.
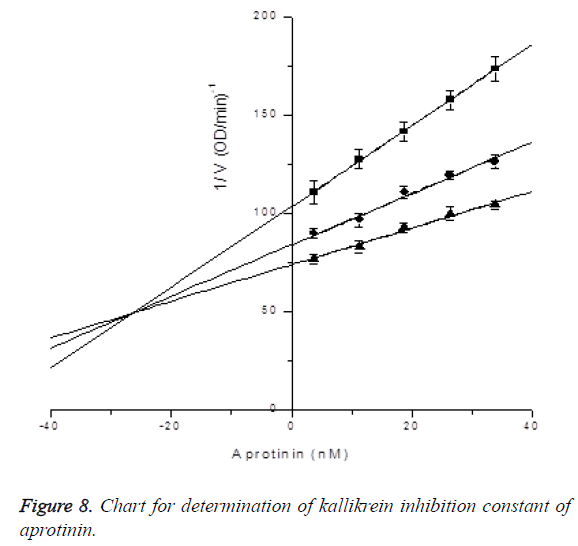
Determination of kallikrein inhibition constant of aprotinin: In the presence of S-2302 with final concentrations of 0.1 mM, 0.2 mM and 0.4 mM, the inhibitory effects of aprotinin with final concentrations of 33.75, 26.25, 18.75, 11.25 and 3.75 nM on kallikrein activity were measured (reacting 21.6, 16.8, 12, 7.2 and 2.4 μl of 1.5 μM aprotinin in 960 μl activity measuring system), and Ki was determined by Dixon plotting. Kallikrein final concentration was 0.175781 μg/ml (after 32-fold diluting 0.54 μg/μl kallikrein into 50 mM Tris-HCl pH 7.4, 10 μl was taken and subjected to activity measurement in the 960 μl system). Through plotting,kallikrein inhibition constant of aprotinin was determined to be Ki=6.42691 ± 3.59332 uM, R2=0.9979; the results are shown in Figure 8.
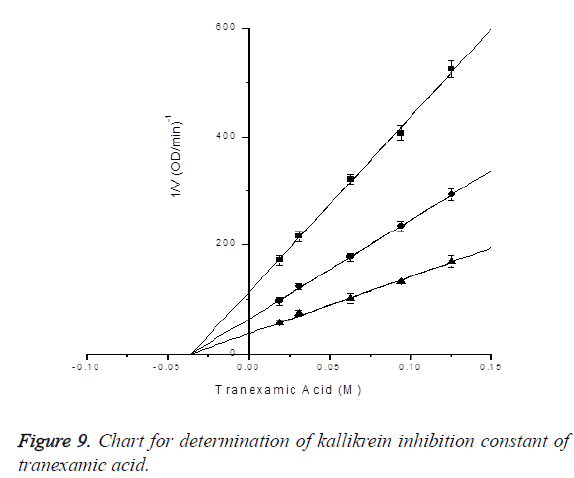
Determination of kallikrein inhibition constant of tranexamic acid: In the presence of S-2302 with final concentrations of 0.1 mM, 0.2 mM and 0.4 mM, the inhibitory effects of tranexamic acid with final concentrations of 0.01875, 0.03125, 0.0625, 0.09375 and 0.125 M on kallikrein activity were measured (reacting 90, 150, 300, 450 and 600 μl of 0.2 M tranexamic acid in 960 μl activity measuring system). 0.54 μg/ μl kallikrein was 30-fold diluted into 50 mM Tris-HCl pH 7.4, 13 μl of which was then subjected to activity measurement in the 960 μl system. Kallikrein dosage was 0.234 μg. Ki was determined by Dixon plotting. Through plotting, kallikrein inhibition constant of tranexamic acid was determined to be Ki=0.03519 ± 0.00144 M, R2=0.99908; the results are shown in Figure 9.
| Proteases | rPI-T1 | Aprotinin | Tranexamic Acid |
|---|---|---|---|
| Plasmin | Ki=12.60 ± 1.54 nM | Ki=4.40 ± 1.58 nM | Ki=42.73 ± 3.23 mM |
| trypsin | Ki=32.26242 ± 2.73167 nM | Ki=0.10604 ± 0.03332 nM | Ki=54.93 ± 4.22 mM |
| Kallikrein | Ki=3.65912 ± 0.46831 uM | Ki=26.42691 ± 3.59332 nM | Ki=35.1 ± 1.44 mM |
Table 1. Summary of experimental results.
Discussion
As can be seen from the Ki values, trypsin, plasmin and kallikrein inhibitory activities of aprotinin are all at nmol level, which decrease by an order of magnitude as well. As for rPIT1, it shows the strongest inhibitory activity on plasmin, followed by trypsin and kallikrein. Its inhibitory activities on the former two proteases are at the same molar level (nmol level). Meanwhile, its kallikrein inhibitory activity is at umol level, which is approximately 1% of the activity of aprotinin. In addition, its inhibitory activity is the strongest on plasmin. Presumably, when used in the treatment of hyperfibrinolytic hemorrhage, rPI-T1 can avoid the risk of severe side effects of aprotinin owing to its weak inhibitory effect on kallikrein. Tranexamic acid and aprotinin had mechanisms of action differing from rPI-T1 (reversible competitive inhibitor), which are non-competitive inhibitors. Their inhibition constants (Ki values) on the above three proteases are all at mmol level. Due to different mechanisms and high working concentrations, they also have some side effects [9-12]. Compared with aprotinin, textilinin-1 has better selectivity and specificity on serine protease system inhibition, i.e. better plasmin reversibility. Therefore, the role of textilinin-1 on complex components in the blood can be better monitored and controlled, that is, the inhibitory effect of textilinin-1 on plasmin-induced bleeding at plasma and injured sites. Thus, in the treatment of hyperfibrinolysis, bleeding and vascular injury, especially the bleeding caused by primary hyperfibrinolysis, textilinin-1 is expected to replace aprotinin as a safer and more reliable hemostatic [13,14]. In addition, textilinin-1 also has a strong inhibitory effect on trypsin, so it can also be used for the treatment and prevention of various types of pancreatitis.
References
- Monikal BS, Françoise B, Jean-Pierre A, Stephane V. Investigation of aprotinin (BPTI) solutions during nucleation. J Crystal Growth 2002; 235: 547-554.
- Veesler S, Ferte N, Costes MS. Temperature and pH Effect on the Polymorphism of Aprotinin (BPTI) in Sodium Bromide Solutions. Crystal Growth Design 2004; 4: 1137-1141.
- Masci PP, Whitaker AN, Sparrow LG, Jersey J, Winzor DJ, Watters DJ, Lavin MF, Gaffney PJ. Textilinins from Pseudonaja textilis textilis. Characterization of two plasmin inhibitors that reduce bleeding in an animal model. Blood Coagul Fibrinol 2000; 11: 385-393.
- Millers EK, Masci PP, Lavin MF, Jersey J, Guddata LW. Crystallization and preliminary X-ray analysis of a Kunitz-type inhibitor, tex tilinin-1 f rom Pseudonaja textilis textilis. Acta Crystallographica F 2006; 62: 642-645.
- Flight SM, Johnsonl LA, Du QS, Warner RL, Trabi M, Gaffney PJ, Lavin MF, Jersey JD, Masci PP. Textilinin-1, an alternative anti-bleeding agent to aprotinin: Importance of plasmin inhibition in controlling blood loss. Brit J Haematol 2009; 145: 207-211.
- Earl STH, Masci PP, Jersey J, Lavin MF, Dixon J. Drug development from Australian elapid snake venoms and the Venomics pipeline of candidates for haemostasis: Textilinin-1 (Q8008), Haempatch™ (Q8009) and CoVase™ (V0801). Toxiconomy 2012; 59: 456-463.
- Kang HM, Kalnoski MH, Frederick M, Chandler WL. The kinetics of plasmin inhibition by aprotinin in vivo. Thrombosis Res 2005; 115: 327-340.
- Wang HY, Li N, Li XN, Wang ZY, Xu M, Xue Y, Xue BZ. Expression and application of textilinin-1, a plasmin inhibitor, in Pichia pastoris. J Snake 2014; 3: 273-277.
- Chauhan S. Comparison of tranexamic acid with aprotinin in pediatric cardiac surgery. Annal Cardiac Anaesthesia 2015; 18: 27-28.
- Muthialu N, Balakrishnan S, Sundar R, Muralidharan S. Efficacy of tranexamic acid as compared to aprotinin in open heart surgery in children. Annal Cardiac Anaesthesia 2015; 18: 23-26.
- Yong Fan, Ru Lin, Lijun Yang, Lifen Ye, Jiangen Yu, Qiang Shu. Retrospective cohort analysis of a single dose of aprotinin use in children undergoing cardiac surgery: a single-center experience. Pediatric Anesthesia 2013; 23: 242-249.
- Charles Marc Samama. Aprotinin and Major Orthopedic Surgery. Transfus Alternat Transfus Med 2004; 6: 28-33.
- Flight SM, Johnson LA, Du QS. Textilinin-1, an alternative antibleeding agent to aprotinin: Importance of plasmin inhibition in controlling blood loss. Br J Haematol 2009; 145: 207-211.
- Flight S, Johnson L, Trabi M, Gaffney P, Lavin M, Jersey J, Masci P. Comparison of Textilinin-1 with Aprotinin as Serine Protease Inhibitors and as Antifibrinolytic Agents. Pathophysiol Haemos Thromb 2005; 34: 188-193.