Research Article - Biomedical Research (2017) Volume 28, Issue 4
The study of factors that affects the fracture healing in diabetic rats
Xi Li, Wei Ren, Fengyao Wang, Yaguang Zhu, Hui Wang, Jinsong Liu*Department of Orthopaedics, First Affiliated Hospital of Kunming Medical University, Kunming, China
- *Corresponding Author:
- Jinsong Liu
Department of Orthopaedics
First Affiliated Hospital of Kunming Medical University, China
Accepted date: September 30, 2016
Abstract
Objective: Our objective is to observe and detect the facture healing, the change of Bone Mineral Density (BMD), and the expressions of Alkaline Phosphatase (ALP), Osteocalcin (OC), Bone Morphogenetic Protein (BMP)-2, Insulin Growth Factor (IGF)-1 and collagen type 1 in local fracture site and serum, and explore the factors that affect the fracture healing in diabetic rats.
Methods: 60 male rats aged 3 months were randomly divided into 2 groups. Group A was the experimental group (diabetic rats with fracture) and group B was the control group (non-diabetic rats with fracture). In group A, Streptozotocin (STZ) was used to damage islet B cells. If there was rat dead or establishment failure during the model establishment then more rats were used for model establishment to make sure there were 5 rats available for detection in each group every time. After that the fracture model was established. At 1, 2, 3, 4, 6, and 8 weeks after the model establishment, the BMD values of lumbar vertebra, hip joint, local fracture site and callus were detected; the blood glucose, calcium, phosphorus and ALP in the serum were detected; OC was detected by radioimmunoassay; serum BMP-1 and IGF-1 were detected by Enzyme-Linked Immunosorbent Assay (ELISA); the expressions of BMP-2, IGF-I and OC in callus tissue were detected by immunohistochemistry.
Results: Compared with the group B, the blood glucose in the group A was significantly increased after 1, 2, 3, 4, 6, and 8 weeks, which were all higher than 13.8 mmol/l; the BMD values of lumbar vertebra, hip joint, local fracture site and callus were decreased, which was most significant after 4 weeks and the difference from group B was most significant after 8 weeks; the blood calcium and phosphorus were decreased, and the difference from group B was most significant after 6 weeks; blood BMP-2, IGF-I, ALP and OC were significantly decreased, and the peak was delayed for a week; immunohistochemistry results showed that BMP-2, IGF-I and OC were decreased in callus tissue; real-time PCR showed that BMP-2, IGF-1 and collagen type 1 in callus tissue were all significantly decreased in the first 3 weeks, the difference was most significant after 2 weeks, BMP-2 and IGF-1 were not significantly different after 4 weeks, the peaks were delayed for 1 week, collagen type 1 was decreased after 4 and 6 weeks with significant differences, however there was no difference after 8 weeks.
Conclusion: The fracture healing is poor in diabetic rats with delayed healing or non-union. The early BMP-2 and IGF-I in blood and local callus tissue were decreased during the fracture healing. During the fracture healing, the number and function of local bone and callus tissue were decreased, and the expression of collagen type 1 was decreased. There was significant loss of calcium and phosphorus. During the fracture healing the BMD of local bone and callus tissue were decreased and there was memorization dysfunction. The decrease of BMP-2 and IGF-1 in serum and local callus tissue can cause the decrease of osteoblast amount and function, decrease collagen type 1, dysfunction of mineralization of callus at late stage, which are the important factors of the poor fracture healing in diabetic rats.
Keywords
Diabetes, Fracture, BMP-2, IGF-1, Collagen type 1, Osteocalcin.
Introduction
Fracture healing is a complex physiological process, including the release of multiple factors, cell migration, angiogenesis, proliferation and maturity of osteoblasts, secretion and maturity of bone matrix, formation and reconstruction of callus and mineralization of callus. Meanwhile, this process involves the regulatory effects of cytokines on differentiation and proliferation of cells, the osteoinduction, and signalling transduction [1-4].
Diabetes Mellitus (DM) is a group of clinical metabolic symptoms, which is one of the 3 non-infectious diseases that significantly affect the health of human. In diabetic patients, there is more risk of fracture due to the muscle weakness and osteoporosis [5]. During fracture healing in diabetic patients, there is obvious difference of osteoinduction and osteoconduction compared to healthy population. As the development of molecular biology, there is more research about the fracture healing in diabetic rats. However, the mechanism of delayed fracture healing still remains unclear, and the theory is also at the early stage. Nowadays, the effects of cytokines on fracture healing in diabetic patients have got more and more attention.
In the previous studies [6-8] it has been shown that Bone Morphogenetic Protein-2 (BMP-2) can induce the mesenchymal cells to transfer to chondroblasts and osteoblasts irreversibly and induce the formation of bone, which plays an important role in the osteogenesis; Insulin-Like Growth Factor-1 (IGF-1) can not only affect the osteoblast to induce differentiation and the synthesis of collagen, but also affect the precursor cells of osteoblasts to stimulate the formation of osteoblasts and further induce the formation of bone matrix [9,10]. However, there has been no study about the effect of BMP-2 and IGF-1 change in local callus on the proliferation and maturity of osteoblasts and secretion of bone matrix. In this study, we established an animal model to observe the changes of the Bone Mineral Density (BMD), the levels of BMP-2, IGF-1, collagen type I and Osteocalcin (OC) in serum and callus, the levels of calcium, phosphorus, Alkaline Phosphates (ALP) in serum, and analyse the factors that affect the fracture healing in diabetic patients. In this study, we focused on the factors that affect the fracture healing in diabetic mice, which can provide valuable guidance for the further therapeutic methods. This can be applied in clinically which is beneficial to the fracture healing of diabetic patients.
Materials and Methods
Animals
60 healthy male SD (Sprague-Dawley) rats aged 3 months were provided by the Experimental Center of Kunming Medical College. The room temperature was maintained at 18-22˚C and the relative humidity was maintained at 40%-70%. The rats were raised in 12 hour light-darkness cycle, and free to get water and food. The rats were raised for 2 weeks before experiment.
Instruments and reagents
Strepozotocin (STZ) was purchased from Sigma-Aldrich Co. LLC., Missouri, USA; OC kit was purchased from Northern Biotechnology Company, Beijing, China; rat BMP-2,IGF-I and OC monoclonal antibodies (rabbit anti rat) and SABC immunohistochemistry kits were purchased from Abcam plc., Cambridge, England; Opticon real-time PCR amplifier was purchased from MJ Research Inc., Florida, USA; Trizol was purchased from Thermo Fisher Scientific Inc., Massachusetts, USA; 10X DNase I Buffer and DNase I (RNase Free,5 μ/μl) were purchased from Takara Biotechnology Co., Ltd., Dalian, China; RNas inhibitor (40 μ/μl) was purchased from Promega Corporation, Wisconsin, USA.
Grouping and model establishment
60 male rats were randomly divided into 2 groups; both were fed with the ordinary forage. Diabetic rats with fracture were included in group A and normal rats with fracture were included in group B. The rats in group A received intraperitoneal injection of STZ (50 mg/kg) with STZ solution in 0.1 mmol/l citric acid-sodium citrate buffer and the rats in group B were injected with the same volume of citric acidsodium citrate buffer [11]. After 24 hours, the blood was collected from the tail to detect blood glucose. After one week, the body weight and blood glucose were detected again. The criteria of diabetes are the decrease of body weight and random non-fasting blood glucose ≥ 13.8 mmol/l [12]. After anaesthesia, the upper section of left tibia was exposed and the tibia was cut by scissor (1 cm from tibial plateau), and then the tibia was fixed by splint. During the experiments, the dead rats or the rats with unsuccessful model establishment were not used. More rats were used for model establishment to make sure that 30 rats in total were used for detection in either group and 5 rats were used for detection in each group at all the stages. In total 78 rats were used.
Detection of BMD
At 1, 2, 3, 4, 6, and 8 weeks after model establishment, 5 SD rats were randomly selected from either group. The rats were sacrificed by intraperitoneal injection of 20 mg/kg 3% pentobarbital sodium. The BMD of lumbar vertebra, hip joint, local fracture site and callus were detected. X-ray was used to detect the BMD according to the tissue absorption rates of different samples. The attenuation degree is related to the bone mineral substance content.
Detection of blood glucose, calcium, phosphorus and ALP in serum
At 1, 2, 3, 4, 6, and 8 weeks after model establishment, 5 SD rats were randomly selected for blood glucose detection. The blood sample was collected from femoral artery, and the blood glucose, calcium, phosphorus and ALP were detected by automatic biochemical analyser (Olympus Co., Tokyo, Japan).
Serum OC detection by radioimmunoassay (RIA)
Aft 1, 2, 3, 4, 6, and 8 weeks after model establishment, 5 SD rats were randomly selected. 2 ml blood was collected from femoral artery, centrifuged at 3000 rpm for 15 minutes to collect the supernatant at 4˚C. 125I-BGP and the OC in the standard competitively bind to the OC antibody. The mixture was incubated at 4˚C for 20 hours to reach the balance, and then the separating reagent was added and incubated at room temperature for 15 minutes. The separating reagent can bind to the OC which binds to antibody to form precipitation; the free OC was centrifuged and discarded. The radio counting of precipitation was detected and the OC level was calculated.
Detection of serum BMP-2 and IGF-I by enzymelinked immunosorbent assay (ELISA)
At 1, 2, 3, 4, 6, and 8 weeks after model establishment, 5 SD rats were randomly selected. 2 ml blood was collected from femoral artery, centrifuged at 3000 rpm for 15 minutes to collect the supernatant. 0.1 ml diluted sample was added in antibody coated well and incubated at 37˚C for 90 minutes, then the liquid in the wells were washed by automatic washer; 0.1 ml biotin labelled antibody was added in each well and incubated at 37˚C for 60 minutes, and then washed by PBS for 5 times, 0.l m1 ABC solution was added and incubated at 37˚C for 90 minutes, again washed by PBS for 5 times; 0.l ml TMB solution was added and incubated at 37˚C in darkness, then the stopping solution was added and the Optical Density (OD) values were read at 450 nm wave length. The BMP-1 and IGF-1 concentrations were calculated according to the standard curve and OD values.
BMP-2, IGF-I and OC detected by immunohistochemistry
After sacrifice, a part of callus tissue was collected for immunohistochemistry. The tissue was fixed, embedded, sliced, dewaxed and dehydrated. Then the primary antibody and secondary antibody were added and developed for 5 minutes. The brown color represents the location of the antigen. The positive slide was used as positive control and PBS was used as negative control. The expression level of BMP-2, IGF-1 and OC were evaluated according to the density and amount of positive staining [13,14].
BMP-2, IGF-I and OC expression detected by RTPCR
The local callus tissue was collected and grinded, homogenized and precipitated to extract the RNA. Then the RNAs were transversely transcripted. Sequences of primers: upstream primer for BMP-2: 5´- TTCTGTCCCTACTGATGAGTTTCTC-3´, downstream primer for BMP-2: 5´- AAGTCACTAGCAGTGGTCTTACCTG-3´; upstream primer for IGF-1: 5´-TTCGTGTGTGGACCAAGGG-3´, downstream primer for IGF-1: 5´-AATGCTGGAGCCATAGCCTG-3´; upstream primer for collagen type 1: 5´- CTCAGGGGCGAAGGCAACAGT-3´; upstream primer for collagen type 1: 5´-ATGGGCAGGCGGGAGGTCT-3’; upstream primer for β-actin: 5’- GTAGCCATCCAGGCTGTGTT-3’; downstream primer for β- actin: 5’-CCCTCATAGATGGGCACAGT-3’. Real-time PCR: denaturation at 95˚C for 4 minutes, then 58˚C for 30 s, 72˚C for 40 s and 76˚C for 2 s to detect the florescence, there were 40 cycles in total. Meanwhile, the melting curve between 53˚C-95˚C was made.
Statistical analysis
SPSS 18.0 was used to analyse the data. All the data were presented as mean ± standard deviation (͞x± S). T-test was used to compare the differences among groups. The significance level was set as α=0.05, P<0.05 was considered as statistically significant.
Results
General characteristics
In group B, the SD rats were normal, the body weights were increased and the hair color was shining, the diet and execration were normal. In group A, after injection of STZ, the SD rats were droop with slow reaction, the body weight was decreased, the water and food intake was increased, the 24 hour urine volume monitored and it was found that the urine volume was increased, and the symptoms were aggravated with the time. It suggests that the rats in the control group were in a good condition; however, the diabetic rats with fracture were in a sick condition compared with group B.
Detection of blood glucose every week
After fracture the blood glucose levels were significantly increased, which were significant at all-time points (P<0.05). The model establishment was successful and stable as shown in Table 1.
| Group A | Group B | T-value | P-value | |
|---|---|---|---|---|
| 1st week | 15.78 ± 3.05* | 4.17 ± 1.11 | 7.9985 | 0 |
| 2nd week | 16.33 ± 1.93* | 3.74 ± 0.43 | 14.2375 | 0 |
| 3rd week | 14.89 ± 1.53* | 3.89 ± 1.32 | 12.1723 | 0 |
| 4th week | 16.43 ± 1.20* | 4.12 ± 1.40 | 14.928 | 0 |
| 6th week | 16.13 ± 1.33* | 4.21 ± 1.83 | 10.4906 | 0 |
| 8th week | 15.35 ± 2.14* | 3.88 ± 0.83 | 11.1739 | 0 |
Table 1: The changes of dynamic blood glucose level in 2 groups (x̄ ± s, ng/ml, n5).
BMD values of lumbar vertebra, hip joint, local fractured bone and callus tissue
In group B, the BMD levels of lumbar vertebra, hip joint and local callus were not changed, although there was slight decrease from 1st week to 4th week, it was obvious in local bone and callus. However, in group A, the BMD of hip joint was decreased, which was slightly decreased at 1st week to 3rd week (P>0.05) and obviously decreased at 4th week with statistical significance (P<0.05). Compared with group B, the BMD levels of the lumbar vertebra, hip joint and callus were obviously decreased, which was most significant at 8th week as shown in Table 2.
| Lumbar spine | Hip joint | Local fracture bone and callus | ||||
|---|---|---|---|---|---|---|
| Group A | Group B | Group A | Group B | Group A | Group B | |
| 1st week | 0.239 ± 0.011 | 0.244 ± 0.027 | 0.193 ± 0.012 | 0.201 ± 0.025 | 0.192 ± 0.015 | 0.204 ± 0.016 |
| 2nd week | 0.235 ± 0.022 | 0.241 ± 0.024 | 0.182 ± 0.035 | 0.193 ± 0.032 | 0.187 ± 0.015 | 0.194 ± 0.027 |
| 3rd week | 0.221 ± 0.026 | 0.238 ± 0.034 | 0.176 ± 0.029 | 0.187 ± 0.021 | 0.177 ± 0.025 | 0.184 ± 0.034 |
| 4th week | 0.197 ± 0.013* | 0.232 ± 0.023 | 0.153 ± 0.017 | 0.173 ± 0.011 | 0.163 ± 0.031 | 0.172 ± 0.015 |
| 6th week | 0.166 ± 0.016* | 0.234 ± 0.022 | 0.124 ± 0.014 | 0.179 ± 0.018 | 0.153 ± 0.012 | 0.188 ± 0.020 |
| 8th week | 0.129 ± 0.018* | 0.233 ± 0.021 | 0.089 ± 0.033 | 0.182 ± 0.015 | 0.142 ± 0.019 | 0.192 ± 0.027 |
Table 2: The BMDs of the lumbar spine, hip joint and local fractured bone and callus in 2 groups at different time points (͞x ± s, g/cm2, n=5).
Results of blood calcium, phosphorus and ALP
Generally, the blood calcium levels in group A were lower than group B at all-time points, which was most significant after 6 weeks (2.33 ± 0.05 mmol/l in group A and 2.61 ± 0.08 mmol/l in group B), and also significant after 1 and 4 weeks (P<0.05), there was no differences at the other time points (P>0.05).There was no difference when compared within the same groups as shown in Table 3. Blood phosphorus levels in group A were lower than group B, the trends in two groups were in accordance with each other and there were significant differences at all-time points (P<0.05) as shown in Table 4. In group A, the blood ALP levels were significantly decreased after fracture, there were significant differences before the 4th week (P<0.01), ALP levels were significantly decreased at 6th and 8th week (p<0.05). The blood ALP level reached peak at 3rd week (232.4 ± 20.5 IU/L), however it reached peak in group B at 2nd week (401.1 ± 10.7I U/L). The difference was most significant at 2nd week as shown in Table 5.
| Group A | Group B | T value | P value | |
|---|---|---|---|---|
| 1st week | 2.12 ± 0.15* | 2.34 ± 0.11 | 2.6447 | 0.0295 |
| 2nd week | 2.48 ± 0.08 | 2.51 ± 0.12 | 0.4651 | 0.6542 |
| 3rd week | 2.32 ± 0.08 | 2.39 ± 0.05 | 1.8196 | 0.1063 |
| 4th week | 2.29 ± 0.07* | 2.41 ± 0.09 | 2.3534 | 0.0464 |
| 6th week | 2.33 ± 0.05* | 2.61 ± 0.08 | 6.6366 | 0.0002 |
| 8th week | 2.18 ± 0.04 | 2.22 ± 0.05 | 1.3963 | 0.2 |
Table 3: The change of serum calcium in 2 groups (͞x ± s, ng/ml, n=5).
| Group A | Group B | T value | P value | |
|---|---|---|---|---|
| 1st week | 3.00 ± 0.11* | 3.32 ± 0.09 | 5.0345 | 0.001 |
| 2nd week | 2.57 ± 0.08* | 2.90 ± 0.07 | 6.9416 | 0.0001 |
| 3rd week | 2.44 ± 0.14* | 2.81 ± 0.12 | 4.4689 | 0.002 |
| 4th week | 2.09 ± 0.10* | 2.29 ± 0.13 | 2.7267 | 0.0026 |
| 6th week | 2.16 ± 0.09* | 2.32 ± 0.11 | 2.5173 | 0.0036 |
| 8th week | 2.19 ± 0.14* | 246 ± 0.08 | 3.7442 | 0.0057 |
Table 4: The change of serum phosphorus in 2 groups (͞x ± s, mmol/l, n=5).
| Group A | Group B | T value | P value | |
|---|---|---|---|---|
| 1st week | 218.4 ± 13.4** | 370.3 ± 19.5 | 14.3556 | 0 |
| 2nd week | 223.6 ± 19.0** | 401.1 ± 10.7 | 20.8113 | 0 |
| 3rd week | 232.4 ± 20.5** | 365.7 ± 16.7 | 11.2728 | 0 |
| 4th week | 209.5 ± 13.6** | 303.4 ± 27.9 | 4.8451 | 0.0013 |
| 6th week | 168.2 ± 11.1* | 242.2 ± 16.2 | 6.5227 | 0.0102 |
| 8th week | 147.9 ± 14.1* | 186.3 ± 13.5 | 4.3987 | 0.0123 |
Table 5: The change of serum ALP in 2 groups (͞x ± s, IU/L, n=5).
The change of serum OC
In group B, the OC level reached peak at 2rd week (2.568 ± 0.097 μg/l), and decreased at 4th, 6th and 8th week; The OC levels in group A were increased from the 1st week to 3rd week, and reached the peak at 3rd week (2.025 ± 0.089 μg/l), and then gradually decreased at 4th, 6th and 8th week; There were significant differences between 2 groups at 1st, 2nd, 3rd and 4th week (P<0.01); there was no statistically difference at the other time points (P<0.05) as shown in Table 6.
| Group | Time/week | |||||
|---|---|---|---|---|---|---|
| 1** | 2** | 3** | 4** | 6* | 8* | |
| Group A | 1.738 ± 0.045 | 1.906 ± 0.098 | 2.025 ± 0.089 | 1.788 ± 0.109 | 1.428 ± 0.079 | 1.337 ± 0.124 |
| Group B | 2.217 ± 0.109 | 2.568 ± 0.097 | 2.557 ± 0.076 | 2.544 ± 0.096 | 1.782 ± 0.107 | 1.571 ± 0.106 |
| T value | 9.0828 | 13.5674 | 10.1645 | 12.5993 | 4.8327 | 3.2075 |
| P value | 0 | 0 | 0 | 0 | 0.0113 | 0.0125 |
Table 6: The change of OC in 2 groups at different time points (͞x ± s, μg/L, n=5).
ELISA results of serum BMP-2 and IGF-I
In group B, serum BMP-2 reached peak at 2nd week (2.88 ± 0.07 ng/ml) and gradually decreased at 4th, 6th and 8th week; in group A, the level was gradually increased from the 1st week to the 3rd week and reached the peak at 3rd week (2.75 ± 0.05 ng/ ml), and then gradually decreased at 4th, 6th and 8th week; there were significant differences between 2 groups at 1st, 2nd and 3rd week (P<0.05 );there was no statistical difference at the other time points (P>0.05) as shown in Table 7. In group B, serum IGF-1 level reached the peak at 2nd week (4.10 ± 0.07 ng/ml), and gradually decreased at 3rd, 4th, 6th and 8th week; serum IGF-1 in group A was gradually increased from the 1st week to 3rd week and reached the peak at 3rd week (4.05 ± 0.04 ng/ml) and then gradually decreased at 4th, 6th, and 8thweek. There were statistical differences between 2 groups at 1st, 2nd and 3rd week (P<0.05), and there was no statistical difference at the other time points (P>0.05) as shown in Table 8.
| Group | Time/week | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 6 | 8 | ||
| Group A | 2.16 ± 0.07* | 2.70 ± 0.04* | 2.75 ± 0.05* | 2.49 ± 0.07 | 2.31 ± 0.08 | 2.16 ± 0.06 | |
| Group B | 2.36 ± 0.15 | 2.88 ± 0.07 | 2.85 ± 0.06 | 2.50 ± 0.10 | 2.23 ± 0.05 | 2.13 ± 0.04 | |
| T-value | 7.2946 | 4.9923 | 2.863 | 0.1832 | 1.8962 | 0.9303 | |
| P-value | 0.0001 | 0.0011 | 0.0211 | 0.8592 | 0.0945 | 0.3795 | |
Table 7: The change of serum BMP-2 in 2 groups (͞x ± s, ng/ml, n=5).
| Group | Time/week | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 6 | 8 | |
| Group A | 3.85 ± 0.08* | 3.91 ± 0.08* | 4.05 ± 0.04* | 4.03 ± 0.03 | 3.91 ± 0.07 | 3.90 ± 0.05 |
| Group B | 4.10 ± 0.07 | 4.22 ± 0.06 | 4.13 ± 0.05 | 4.00 ± 0.04 | 3.90 ± 0.08 | 3.85 ± 0.04 |
| T-value | 5.2588 | 6.9318 | 2.7937 | 1.3416 | 0.2104 | 1.7461 |
| P-value | 0.0008 | 0.0001 | 0.0234 | 0.2165 | 0.8387 | 0.1189 |
Table 8: The change of serum IGF-I in 2 groups (͞x ± s, ng/ml, n=5).
Immunohistochemistry results
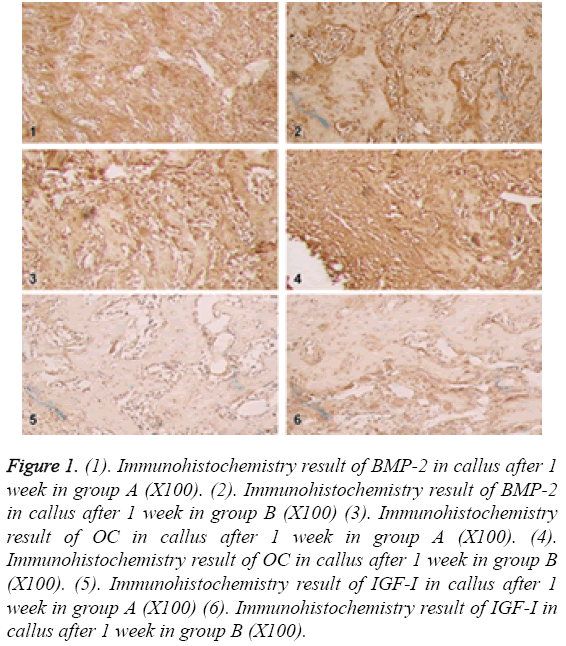
In the 1st week, BMP-2 and OC were only expressed in some mesenchymal cells, a few of osteoblasts and chondroblasts in group A; in group B, BMP-2 and OC were expressed in osteoblasts, chondroblasts, cartilage matrix, fibroblasts and granulation tissue, which were significantly different between 2 groups. IGF-1 was weakly expressed in a few of mesenchymal cells and granulation tissue in group A; in group B, IGF-I was expressed in granulation tissue, mesenchymal tissue and callus tissue, which was significantly different between 2 groups as shown in Figure 1.
Figure 1: (1). Immunohistochemistry result of BMP-2 in callus after 1 week in group A (X100). (2). Immunohistochemistry result of BMP-2 in callus after 1 week in group B (X100) (3). Immunohistochemistry result of OC in callus after 1 week in group A (X100). (4). Immunohistochemistry result of OC in callus after 1 week in group B (X100). (5). Immunohistochemistry result of IGF-I in callus after 1 week in group A (X100) (6). Immunohistochemistry result of IGF-I in callus after 1 week in group B (X100).
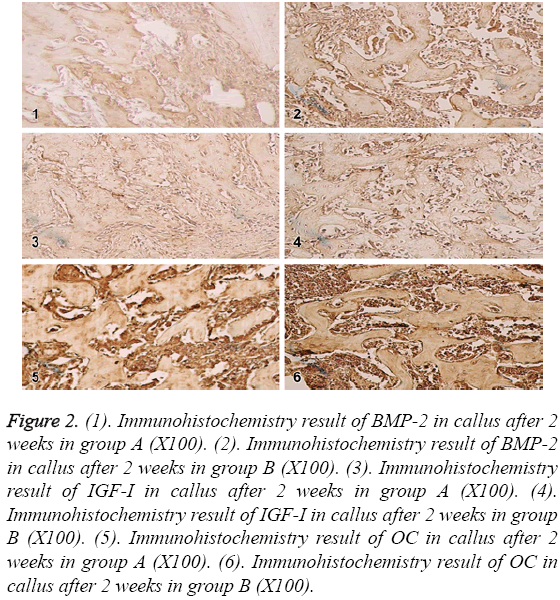
In the 2nd week, BMP-2,IGF-I and OC were expressed in a few of fibroblasts, osteoblasts, mature chondrocytes and bone matrix in group A; in group B, the expressions reached the peak and showed strongly positive in fibroblasts, osteoblasts, mature chondrocytes and bone matrix. The difference was most significant at 2nd week as shown in Figure 2.
Figure 2: (1). Immunohistochemistry result of BMP-2 in callus after 2 weeks in group A (X100). (2). Immunohistochemistry result of BMP-2 in callus after 2 weeks in group B (X100). (3). Immunohistochemistry result of IGF-I in callus after 2 weeks in group A (X100). (4). Immunohistochemistry result of IGF-I in callus after 2 weeks in group B (X100). (5). Immunohistochemistry result of OC in callus after 2 weeks in group A (X100). (6). Immunohistochemistry result of OC in callus after 2 weeks in group B (X100).
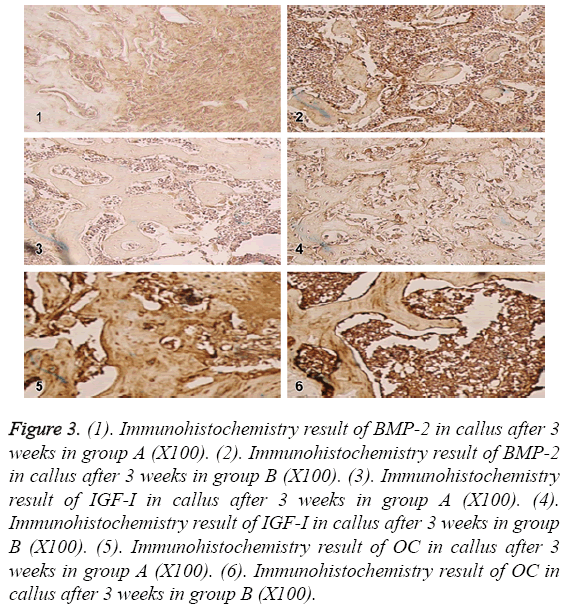
In the 3rd week, BMP-2,IGF-1 and OC reached the peak in osteoblasts, mature chondrocytes and bone matrix in group A; in group B, the expressions in osteoblasts, mature chondrocytes and bone matrix were decreased compared to before, but still positive. The expressions in group A were lower than group B as shown in Figure 3.
Figure 3: (1). Immunohistochemistry result of BMP-2 in callus after 3 weeks in group A (X100). (2). Immunohistochemistry result of BMP-2 in callus after 3 weeks in group B (X100). (3). Immunohistochemistry result of IGF-I in callus after 3 weeks in group A (X100). (4). Immunohistochemistry result of IGF-I in callus after 3 weeks in group B (X100). (5). Immunohistochemistry result of OC in callus after 3 weeks in group A (X100). (6). Immunohistochemistry result of OC in callus after 3 weeks in group B (X100).
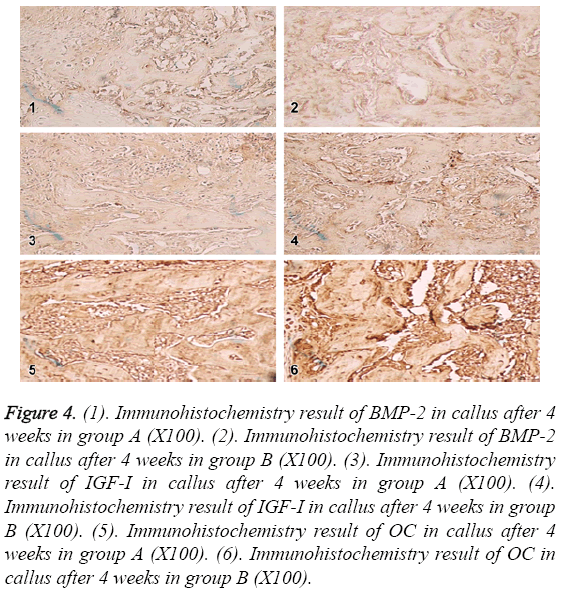
In the 4th week, BMP-2, IGF-1 and OC in group A and B were positive in the same location with lower expression. BMP-2 and IGF-1 expression were decreased more than OC. BMP-2 and IGF-1 expressions were not different. OC in group A was lower than group B as shown in Figure 4.
Figure 4: (1). Immunohistochemistry result of BMP-2 in callus after 4 weeks in group A (X100). (2). Immunohistochemistry result of BMP-2 in callus after 4 weeks in group B (X100). (3). Immunohistochemistry result of IGF-I in callus after 4 weeks in group A (X100). (4). Immunohistochemistry result of IGF-I in callus after 4 weeks in group B (X100). (5). Immunohistochemistry result of OC in callus after 4 weeks in group A (X100). (6). Immunohistochemistry result of OC in callus after 4 weeks in group B (X100).
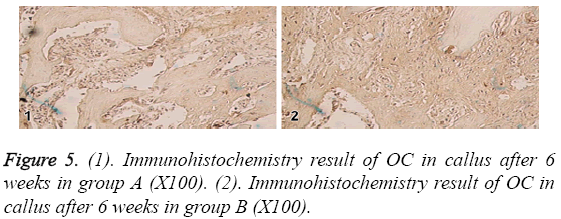
In the 6th week, BMP-2 and IGF-I were positive in the residual osteoblasts, osteoclasts and bone matrix in group A and B. The positive levels were slightly lower than the 4th week, however OC level was significant lower than the 4th week. BMP-2, IGFI and OC were not statistically different among each other as shown in Figure 5.
In the 8th week, BMP-2, IGF-I and OC in the residual osteoblasts, osteoclasts and bone matrix were positive in group A; in group B, they were weakly positive in a few of osteoclasts and bone matrix, showing the same trend. The peak was at the 3rd week in group A and 2nd week in group B.
Real-time PCR results
In the first 3 weeks, BMP-2 mRNA level in group A was significantly lower than group B, group B reached the peak at 2nd week and gradually decreased at 4th, 6th and 8th week; the expression in group A was gradually increased from the 1st week to the 3rd week, and decreased at 4th, 6th and 8th week; there were significant differences between group A and B at 1st, 2nd and 3rd week (P<0.05); there was no statistical difference at the other time points (P>0.05) as shown in Table 9.
| 1stweek | 2ndweek | 3rdweek | 4thweek | 6thweek | 8thweek | |
|---|---|---|---|---|---|---|
| Group A | 1 | 1.082 ± 0.012* | 1.107 ± 0.009* | 1.059 ± 0.014 | 1.023 ± 0.008 | 0.978 ± 0.012 |
| Group B | 1.156 ± 0.008 | 1.313 ± 0.004 | 1.176 ± 0.006 | 1.054 ± 0.007 | 1.017 ± 0.006 | 0.969 ± 0.014 |
| T value | - | 40.8354 | 14.246 | 0.7143 | 1.3416 | 1.0914 |
| P value | - | 0 | 0 | 0.4954 | 0.2165 | 0.3069 |
Table 9: The expression of BMP-2 in local callus in 2 groups (x̄ ± s, n=5).
IGF-1 mRNA in group A were significantly lower than group B in the first 3 weeks, it reached the peak at 2nd week in group B and gradually decreased at 4th, 6th and 8th week in groups B; the expression in group A was gradually increased from the 1st week to the 3rd week, and reached the peak at 3rd week, then gradually decreased at 4th, 6th and 8th week; there were significant differences between group A and B at the 1st, 2nd and 3rd week (P<0.05); there was no statistical difference at the other time points (P>0.05) as shown in Table 10.
| 1stweek | 2ndweek | 3rdweek | 4thweek | 6thweek | 8thweek | |
|---|---|---|---|---|---|---|
| Group A | 1 | 1.084 ± 0.05* | 1.117 ± 0.006* | 1.063 ± 0.007 | 1.033 ± 0.006 | 0.982 ± 0.004 |
| Group B | 1.158 ± 0.006 | 1.321 ± 0.008 | 1.188 ± 0.004 | 1.058 ± 0.005 | 1.027 ± 0.004 | 0.977 ± 0.005 |
| T value | - | 56.1744 | 22.0612 | 1.2994 | 1.8605 | 1.7461 |
| P value | - | 0 | 0 | 0.2299 | 0.0999 | 0.1189 |
Table 10: The expression of IGF-I in local callus in 2 groups (͞x ± s, n=5).
Type I collagen mRNA in group A were significantly lower than group B in the first 4 weeks, it reached the peak at 2nd week in group B and gradually decreased at 6th and 8th week in groups B; the expression in group A was gradually increased from the 1st week to the 3rd week, and reached the peak at 3rd week, then gradually decreased at 4th, 6th and 8th week; there were significant differences between group A and B at the 1st, 2nd, 3rd and 4th week (P<0.05); there was no statistical difference at the other time points (P>0.05) as shown in Table 11.
| 1stweek | 2ndweek | 3rdweek | 4thweek | 6thweek | 8thweek | |
|---|---|---|---|---|---|---|
| Group A | 1 | 1.146 ± 0.047* | 1.257 ± 0.089* | 1.046 ± 0.087 | 0.725 ± 0.084 | 0.612 ± 0.124 |
| Group B | 1.449 ± 0.109 | 1.801 ± 0.097 | 1.789 ± 0.076 | 1.776 ± 0.096 | 1.014 ± 0.107 | 0.803 ± 0.106 |
| T value | - | 13.5822 | 10.1645 | 12.5993 | 4.7505 | 2.1123 |
| P value | - | 0 | 0 | 0 | 0.064 | 0.06776 |
Table 11: The expression of Type I collagen in local callus in 2 groups (͞x ± s, n=5).
Discussion
Up to our knowledge, the factor that affects the fracture healing of diabetic patients has not been explored thoroughly. However, the clinical studies have shown that [15-17], diabetic patients are more liable to have delayed fracture healing even not healing. Some studies have shown that the low insulin status and high blood glucose status in diabetic patients can cause dysfunction of metabolism and decreased the expression of multiple cytokine to cause delayed fracture healing [18,19].
The factors that determine the healing of fracture include the local blood supply, the proliferation and maturity of osteoblasts at fracture site, secretion and maturity of bone matrix, the precipitation and mineralization of calcium and phosphorus in local callus [20,21]. In this study, generally the blood calcium and phosphorus in group A were lower than group B. This is may be due to the high blood glucose induced osmotic dieresis. In diabetic rats high blood glucose can increase the excretion of calcium and phosphorus, and the re-absorption of calcium by renal tubule is decreased, the active transport of calcium is also decreased. These factors cause the low calcium and phosphorus level in rats.
In group A, the BMD levels of lumbar vertebra and hip joint were decreased, which may be related to the loss of calcium and phosphorus. There are some factors that decrease the vitamin D3 in the blood of diabetic patients, such as increase of osteoclasts induced by high glucose and precipitation dysfunction of calcium and phosphate. The bone matrix composed of collagen is an important place of bone minimization. Insulin can promote the amino acid up taken by osteocytes and synthesis of proteins. In diabetic patients, not only the synthesis of collagen will be decreased, but also glycosylation by high glucose can decreased the collagen tenacity and increase bone fragility which further affects the combination of mineral substances and collagen. During the development of osteoblasts, high glucose can stimulate cell proliferation, but inhibit the calcium uptake, which may be related to the change of bone structure in diabetic patients. Also, the long-term high glucose concentration increases the advanced glycation end-products; accumulation of these products affects the adhesion of osteoblasts to the collagen to further change the function, differentiation and proliferation of osteoblasts [22]. Meanwhile, it can induce the production of cytokines, such as interleukin-6 [23], tumor necrosis factor-α and nitric oxide [24] to cause the differentiation, maturity and activity increase of osteoblasts, increase the bone turnover and the severe imbalance of bone formation and bone absorbance, finally cause the decrease of BMD. At 4th week, the BMD in group A was significantly decreased compared with group B, it was considered to be related to the loss of calcium and phosphorus was increased with the time, and blood vitamin D3 was further decreased. In group B, the BMD levels in local fracture bone and callus were slightly increased at 4th week, it was considered to be related to increased callus density, the precipitation of mineral substances, and the increased activity of rats. In group A, the local BMD level was still decreasing, it was considered to be related to the huge loss of calcium and phosphorus, the increased activity of osteoclasts, and the binding difficulty of calcium and phosphorus to collagen caused by high glucose, and further cause the fracture healing difficulty.
ALP is the marker of early stage osteoblast differentiation and OC mainly appears in the mineralization stage which is the marker of osteoblast maturity. The expression levels of them can reflect the bone formation process [25]. Collagen type I is the main organic component in bone matrix, which is the basis to connect the fractured terminal of bone and the implantation of further mineral substance adhesion [26,27]. In this study, after model establishment increases of ALP and OC in group B were obviously lower than group B and the peak was 1 week after group B. Local OC and collagen type in group B reached the peak at 2nd week and dropped at 4th week. The 4th week is the primitive callus formation stage, the new vessels grow into the fibrous connection, the big amount of osteoblasts proliferate, synthesize the secrete bone matrix, which is the process of osteoid tissue formation. In group A, the local OC and collagen type I in group A were obviously lower than group B, the peak was 1 week after groups, which was in accordance with the change of local BMP-2 and IGF-1. It suggests that they are highly correlated during the fracture healing in diabetic rats. The decrease of local BMP-2 and IGF-1 can cause the decrease of local OC and collagen type I to further cause the fracture healing dysfunction.
We also found that diabetes can cause the decrease of multiple systemic and local cytokines. At present, multiple bonederived growth factors can be isolated from bone matrix, osteoblasts and bone [28,29] among these BMP-2 has the strongest osteogenesis effect and plays an most important bone tissue repair [30]. IGF-I can induce the mitosis of multiple cells [31], and also induce the differentiation of the cells [32]. The two factors both play important roles in the process of fracture healing. In this study, local levels of BMP-2 and IGF-1 are in accordance in the serum levels. In the early stage of fracture healing, BMP-2 and IGF-1 play important roles in stimulating mesenchymal cells to transfer to osteoprogenitor cells and then osteoblasts, and also the differentiation of chondroblast [33,34]. The first 2 weeks after fracture is the differentiation peak of osteoblasts and chondroblasts [33,34]. As the differentiation from osteoblasts to osteocytes and the maturity of chondrocytes, local BMP-2 and IGF-I are decreased. After 4 weeks as the increase of callus reconstruction, the expression levels of BMP-2 and IGF-1 are increased which further increase the construction process of osteoblasts in callus. At 8 weeks after fracture in normal rats, the fracture is almost healed; sometimes there is only slight expression in osteoclasts and bone matrix. BMP-2 and IGF-I in diabetic rats were obviously lower than normal bone fracture. In diabetic rats, BMP-2 and IGF-I reached the peak at 3rd week, which was 1 week later than the control group. In the diabetic rats, BMP-2 and IGF-1 were still expressed in some osteoblasts, chondrocytes, bone matrix and osteoclasts, which further suggests that fracture healing of diabetic fracture is delayed.
In conclusion, the fracture healing is poor in diabetic rats with delayed healing or non-union. The early BMP-2 and IGF-I in blood and local callus tissue were decreased during the fracture healing. During the fracture healing, the number and function of local bone and callus tissue were decreased, and the expression of collagen type I was decreased. There was significant loss of calcium and phosphorus. During the fracture healing the BMD of local bone and callus tissue were decreased and there was memorization dysfunction. The decrease of BMP-2 and IGF-1 in serum and local callus tissue can cause the decrease of osteoblast amount and function, decrease collagen type I, dysfunction of mineralization of callus at late stage, which are the important factors of the poor fracture healing in diabetic rats.
Acknowledgements
This work was supported by the Doctoral Fund of First Affiliated Hospital of Kunming Medical University (No. 201313503).
References
- Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res 2002; 5: 13.
- Gomez-Barrena E, Rosset P, Lozano D, Stanovici J, Ermthaller C. Bone fracture healing: cell therapy in delayed unions and non-unions. Bone 2015; 70: 93-101.
- Rose FR, Oreffo RO. Bone tissue engineering: hope vs. hype. BiochemBiophys Res Commun 2002; 292: 1-7.
- Deng Z, Xu B, Jin J. Strategies for management of spinal metastases: A comprehensive review. Cancer Trans Med 2015; 1: 94.
- Regan DK, Manoli A, Hutzler L. Impact of diabetes mellitus on surgical quality measures after ankle fracture surgery: implications for value-based compensation and pay for performance. Journal OrthopTraum 2015; 29: e483-e486.
- Dang PN, Dwivedi N, Yu X. Guiding chondrogenesis and osteogenesis with mineral-coated hydroxyapatite and BMP-2 incorporated within high-density hMSC aggregates for bone regeneration. ACS BiomatSciEng 2015; 2: 30-42.
- Ren X, Bischoff D, Weisgerber DW, Lewis MS, Tu V. Osteogenesis on nanoparticulate mineralized collagen scaffolds via autogenous activation of the canonical BMP receptor signaling pathway. Biomat 2015; 50: 107-114.
- Fan J, Im CS, Guo M, Cui ZK, Fartash A. Enhanced osteogenesis of adipose-derived stem cells by regulating bone morphogenetic protein signaling antagonists and agonists. Stem Cells Transl Med 2016; 5: 539-551.
- Feng R, Ma X, Ma J, Jia H. Positive effect of IGF-1 injection on gastrocnemius of rat during distraction osteogenesis. J Orthop Res 2015; 33: 1424-1432.
- Vahabzadeh S, Bandyopadhyay A, Bose S, Mandal R, Nandi SK. IGF-loaded silicon and zinc doped brushite cement: physico-mechanical characterization and in vivo osteogenesis evaluation. IntegrBiol (Camb) 2015; 7: 1561-1573.
- Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 2001; 50: 537-546.
- Wilkinson-Berka JL, Kelly DJ, Koerner SM, Jaworski K, Davis B. ALT-946 and aminoguanidine, inhibitors of advanced glycation, improve severe nephropathy in the diabetic transgenic (mREN-2)27 rat. Diabetes 2002; 51: 3283-3289.
- Hughes-Fulford M, Li CF. The role of FGF-2 and BMP-2 in regulation of gene induction, cell proliferation and mineralization. J OrthopSurg Res 2011; 6: 8.
- Vermeulen AH, Vermeer C, Bosman FT. Histochemical detection of osteocalcin in normal and pathological human bone. J HistochemCytochem 1989; 37: 1503-1508.
- Weber DR, Haynes K, Leonard MB. Type 1 diabetes is associated with an increased risk of fracture across the life span: A population-based cohort study using The Health Improvement Network (THIN). Diabetes Care 2015; 38: 1913-1920.
- Nicodemus KK, Folsom AR. Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care 2001; 24: 1192-1197.
- Schwartz AV, Sellmeyer DE, Ensrud KE, Cauley JA, Tabor HK. Older women with diabetes have an increased risk of fracture: A prospective study. J ClinEndocrinolMetab 2001; 86: 32-38.
- Shah VN, Shah CS, Snell-Bergeon JK. Type 1 diabetes and risk of fracture: meta-analysis and review of the literature. Diabet Med 2015; 32: 1134-1142.
- Shah VN. Type 1 Diabetes is associated with an increased risk of fracture across the life span: A population-based cohort study using the Health Improvement Network (THIN). Diabetes Care 2015; 38: 1913-1920.
- Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol 2015; 11: 45-54.
- Jiao H, Xiao E, Graves DT. diabetes and its effect on bone and fracture healing. CurrOsteoporos Rep 2015; 13: 327-335.
- Sahin G, Polat G, Bagis S. Study of axial bone mineral density in postmenopausal women with diffuse idiopathic skeletal hyperostosis related to type 2 diabetes mellitus. J Womens Health (Larchmt) 2002; 11: 801-804.
- Takagi M, Kasayama S, Yamamoto T, Motomura T, Hashimoto K. Advanced glycation end products stimulate interleukin-6 production by human bone-derived cells. J Bone Miner Res 1997; 12: 439-446.
- Tzaphlidou M, Kafantari H. Influence of nutritional factors on bone collagen fibrils in ovariectomized rats. Bone 2000; 27: 635-638.
- Kinoshita Y, Arai M, Ito N, Takashi Y, Makita N. High serum ALP level is associated with increased risk of denosumab-related hypocalcemia in patients with bone metastases from solid tumors. Endocr J 2016; 63: 479-484.
- Vauhkonen M, Peltonen J, Karaharju E, Aalto K, Alitalo I. Collagen synthesis and mineralization in the early phase of distraction bone healing. Bone Miner 1990; 10: 171-181.
- Mohan S, Wergedal JE, Das S. Conditional disruption of miR17-92 cluster in collagen type I-producing osteoblasts results in reduced periosteal bone formation and bone anabolic response to exercise. PhysiolGenom 2015; 47: 33-43.
- Tanaka H, Quarto R, Williams S, Barnes J, Liang CT. In vivo and in vitro effects of insulin-like growth factor-I (IGF-I) on femoral mRNA expression in old rats. Bone 1994; 15: 647-653.
- Martino MM, Briquez PS, Maruyama K, Hubbell JA. Extracellular matrix-inspired growth factor delivery systems for bone regeneration. Adv Drug Deliv Rev 2015; 94: 41-52.
- SaIto Y, Yoshizawa T, Takizawa F. A cell line with characteristics of the periodontal ligament fibroblasts is negatively regulated for mineralization and Runxz/Cbfa1/Osfz activity, part of which can be overcome by bone morphogenetic protein-2. J Cell Sci 2002; 115: 4191-4200.
- Strong DD, Merriman HL, Landale EC, Baylink DJ, Mohan S. The effects of the insulin-like growth factors and transforming growth factor beta on the Jun proto-oncogene family in MC3T3-E1 cells. Calcif Tissue Int 1994; 55: 311-315.
- Boguslawski G, Hale LV, Yu XP. Activation of osteocalcin transcription involves interaction of protein kinase A- and protein kinase C-dependent pathways. J BiolChem 2000; 275: 999-1006.
- Wang X, Schwartz Z, Gittens RA, Cheng A, Olivares-Navarrete R. Role of integrin α2 β1 in mediating osteoblastic differentiation on three-dimensional titanium scaffolds with submicron-scale texture. J Biomed Mater Res A 2015; 103: 1907-1918.
- Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. Biology and clinical applications. J Bone Joint Surg Am 2002; 84-84A: 1032-44.