Research Article - Biomedical Research (2018) Volume 29, Issue 4
The role of survivin in oral squamous cell carcinoma
Liang Rong, Hongyan Guo and Ke Liu*
Department of Stomatology, Jinling Hospital, Nanjing, JiangSu Province, PR China
Accepted date: November 21, 2017
DOI: 10.4066/biomedicalresearch.29-17-3461
Visit for more related articles at Biomedical ResearchAbstract
Tumor samples were collected from 30 patients who were suffered with squamous cell carcinoma of the oral cavity. The patients were treated by radical surgical resection between 2012 and 2015 at the Jinling hospital. There were 24 patients who were positive for survivin (80.0%) and 6 patients who were negative for survivin (20.0%). Compared with normal mucosa, there were significantly up-regulated survivin and caspase 9 in OSCC which were proved by Flow Cytometry (FCM) and western blot, respectively. In conclusion, survivin and Caspase 9 might be prognostic markers for Oral Squamous Cell Carcinoma (OSCC).
Keywords
Oral squamous cell carcinoma, Surviving, Caspase 9, Proved by flow cytometry
Introduction
Oral Squamous Cell Carcinoma (OSCC) is the sixth most common cancer all over the world, whose incidence is about 264,000 cases and 128,000 deaths every year [1]. The overall 5 y survival rate of OSCC is still less than 50%, it has not been improved significantly during the past 3 decades [2,3]. OSCC often leads to dysfunctions of chewing, swallowing, speech as well as the loss of esthetics. What’s more, although there are more than 80% of early-stage OSCC patients that can be cured by therapy, there are still about 70% of patients with progressive disease cannot be cured [4]. Consequently, it is urgently needed for precise early detection of OSCC patients.
Survivin (baculoviral inhibitor apoptosis protein repeatcontaining 5, BIRC5) is a cell cycle protein, whose expression is increased during the phase of G1 and reaches the maximum during the phase of G2/M [5]. Up to now, two common functions of survivin have been identified: an antiapoptotic property, which is remained controversial [6,7] and cell cycle control [8].
Survivin is found to be significantly over-expressed in human melanoma cells when compared with normal melanocytes [9], which seem to be a factor with a great chance for promoting the early-step melanoma formation in vivo [10]. As acknowledged, nonmelanoma skin cancers include SCC, basal cell carcinoma, and other less frequent tumors, etc. Survivin is reported to be over-expressed in human and mouse SCCs when compared to that in normal skin [11,12], and is tightly correlated with tumor aggressiveness and lymph node metastasis [13]. Meanwhile, Khan et al. found the overexpression of survivin in OSCC [14].
Over the past 15 years, survivin has been becoming an exciting area for scientific research, mainly on account of its highest expression level in various types of human cancers. In sum, survivin is poorly, or not, expressed in normal adult tissues, however, its expression was significantly increased in tumor cells, which points to survivin as a putative “tumor-specific antigen”. Under the stimulation of apoptosis involving mitochondria, survivin can form a complex with XIAP, elevate the stability of XIAP and its inhibitory activity against caspases [15,16]. Common cancer treatments currently induce apoptosis by downregulating the expression of survivin [17].
Therefore, early detection of OSCC is urgently needed. We aimed to carry out experiments to figure it out.
Materials and Methods
Patients
Paraffin-embedded tumor samples were collected from 30 patients with squamous cell carcinoma of the oral cavity. Their mean age was 67 y old ranging at 45-83 y old. There were 27 male patients (90.0%) and 3 female patients (10.0%) who were enrolled in the present research. The patients were treated by radical surgical resection between 2012 and 2015 at the Jinling hospital.
Examination of the expression level of survivin in OSCC and normal mucosa
Tissues were minced into the volume of about l mm3, followed by washes with PBS and saline solution (0.67-0.70%). After that, the obtained suspension was centrifuged at the speed of 1500 r/min for 5 min, and the cells were re-suspended by addition of l ml PBS to get cell suspension, next, which was washed with 10 ml PBS, centrifuged at the speed of 1500 r/min for 5 min, and the cells were re-suspended by addition of l ml PBS to get single cell suspension. Afterwards, 100 μl primary antibody was added into single cell suspension and incubated at room temperature for 30 min; then 100 μl survivin secondary antibody FITC-IgG was added and incubated at room temperature for 1 h in dark place. At last, the survivin protein level was detected by FCM.
Western blot
Tissues were extracted with RIPA. And protein concentration was quantified with the method of BCA. Protein samples were first separated by 8% SDS-PAGE, and then transferred to PVDF membranes. After the incubation and blockage of membranes with 5% BSA for 1 h at room temperature, caspase-9 antibody was added and incubated overnight at the temperature of 4°C. On the next day, the secondary antibody was added and incubated for 1 h at room temperature. At last, the Supersignal West Femto/Pico HRP sensitive chemiluminiscence substrate was adopted for color development. GAPDH was adopted as an internal control.
Statistical analyses
The statistical analyses in this study were carried out by IBM SPSS software (version 18/19; IBM Inc.). A significance level of 5% was established for all of the statistical tests.
Results
Clinical characteristics of patients
As presented in Table 1., for the characteristics of tobacco consumption, there were 19 smokers (63.3%) and 7 nonsmokers (23.3%) with 4 patients not specified (13.3%); regarding alcohol consumption, there were 18 patients who presented regularly (60.0%) and 5 patients not regularly (16.7%) with 7 patients not specified (23.3%). There were 17 patients at I and II TNM grade (56.7%) and 13 patients at the III and IV TNM grade (43.3%). Meanwhile, there were 24 patients who were positive for survivin (80.0%) and 6 patients who were negative for survivin (20.0%).
| Characteristics | Case (n) | Percent (%) |
|---|---|---|
| Age (y) | ||
| Average | 67 | |
| Range | 45-83 | |
| Sex | ||
| Male | 27 | 90 |
| Female | 3 | 10 |
| Tobacco consumption | ||
| Smoker | 19 | 63.3 |
| Non-smoker | 7 | 23.3 |
| Not specified | 4 | 13.3 |
| Alcohol consumption | ||
| Regularly | 18 | 60 |
| Not regularly | 5 | 16.7 |
| Not specified | 7 | 23.3 |
| TNM Grade | ||
| I and II | 17 | 56.7 |
| III and IV | 13 | 43.3 |
| Survivin | ||
| Positive | 24 | 80 |
| Negative | 6 | 20 |
Table 1. Clinical characteristics of patients included in the study.
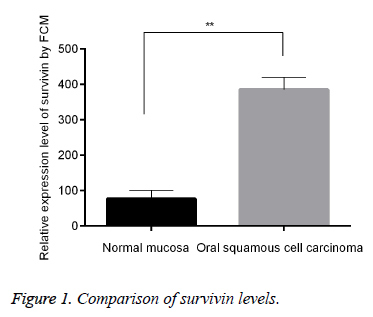
Up-regulated survivin in OSCC than in normal mucosa
FCM was applied for the examination of survivin protein level between OSCC and normal mucosa. Results showed that, there was obviously higher survivin level when compared with that of normal mucosa (p<0.01, Figure 1). Moreover, our result was in consistent with previous studies about the changes of survivin in tumor tissues, for instance, SCC [11,12] and OSCC [14].
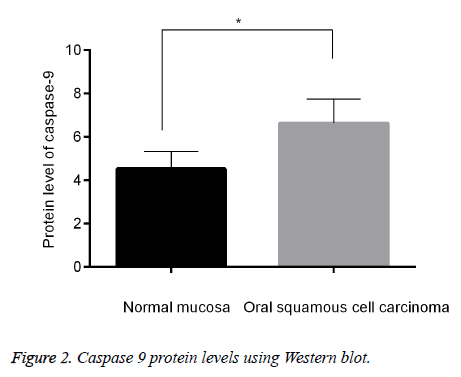
Down-regulated caspase 9 in OSCC than in normal mucosa
As we all well known, dysfunction of the apoptotic system leads to the pathogenesis of a large quantity of diseases, including cancer [18]. Caspases are a class of cysteine proteases which can be divided into mainly two different groups according to their corresponding functions in apoptosis (caspase-3/6/7/8/9) and inflammation (caspase-1/4/5/12) [19].
Since caspase 9 was one of the downstream molecules of survivin [20], we were eager to know the expression changes of caspase 9 between OSCC and normal mucosa. Western blot was applied for the detection of caspase 9 protein level in tissues, we found that, it was significantly higher in OSCC than in normal mucosa (p<0.05, Figure 2). The result was in consistent with a reported study which showed that expression level of caspase-9 was higher in OSCC cells than in the adjacent normal cells [21].
Discussion
There were 24 patients who were positive for survivin (80.0%) and 6 patients who were negative for survivin (20.0%). In the present study, we found that, compared with normal mucosa, there were significantly up-regulated protein levels of survivin and caspase 9 in OSCC.
Our result was similar with previous studies. For instance, one study has found that, significant over-expression of survivin was in laryngeal cancer cells when compared with that in noncancerous cells [22]. Survivin isoforms are regulated following keratinocyte apoptosis, suggesting the potential role in the maintenance of epidermal homeostasis [23]. Survivin protects against apoptosis and regulates the progression of cell cycle, therefore, it is conceivable that, survivin plays an important role in the pathogenesis of psoriasis. Upregulation of survivin has been observed in the epidermis of psoriatic patients who were treated with narrow-band UVB combined with etanercept (tumor necrosis factor-a inhibitor), suggesting the survivin-dependent increased risk of skin cancer formation in psoriatic patients who undergo this type of combined therapy [24].
There was significantly elevated expression of caspases in the tumor tissues of multiple cancer types, including breast carcinomas [25], non-small cell lung carcinoma [26], and oral carcinoma [21]. As acknowledged, caspase cascades play important roles in the process of apoptosis and are tightly associated with the development and prognosis of cancers. However, the effect of expression and activation of various caspases in tumorigenesis is still a double-edged sword in cancers. In the current study, it was suggested that apoptosisrelated caspase-9 was elevated to inhibit tumor cell apotosis.
Taken together, targeting survivin and caspase-9 in its different cellular locations and subcellular locations will provide a strong therapeutic strategy in the future [27].
Funding
The work was supported by Jinling Hospital Management Project (2015018).
References
- Min R, Siyi L, Wenjun Y. Toll-like receptor-9 agonists increase cyclin D1 expression partly through activation of activator protein-1 in human oral squamous cell carcinoma cells. Cancer Sci 2012; 103: 1938-1945.
- Dos Reis PP, Bharadwaj RR, Machado J. Claudin 1 overexpression increases invasion and is associated with aggressive histological features in oral squamous cell carcinoma. Cancer 2008; 113: 3169-3180.
- Marsh D, Suchak K, Moutasim KA, Vallath S, Hopper C, Jerjes W, Upile T, Kalavrezos N, Violette SM, Weinreb PH, Chester KA, Chana JS, Marshall JF, Hart IR, Hackshaw AK, Piper K, Thomas GJ. Stromal features are predictive of disease mortality in oral cancer patients. J Pathol 2011; 223: 470-481.
- Sasahira T, Kurihara M, Bhawal UK, Ueda N, Shimomoto T, Yamamoto K, Kirita T, Kuniyasu H. Downregulation of miR-126 induces angiogenesis and lymphangiogenesis by activation of VEGF-A in oral cancer. Br J Cancer 2012; 107: 700-706.
- Otaki M, Hatano M, Kobayashi K, Ogasawara T, Kuriyama T, Tokuhisa T. Cell cycle-dependent regulation of TIAP/m-survivin expression. Biochim Biophys Acta 2000; 1493: 188-194.
- Tamm I, Wang Y, Sausville E. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res 1998; 58: 5315-5320.
- Dohi T, Okada K, Xia F, Wilford CE, Samuel T, Welsh K, Marusawa H, Zou H, Armstrong R, Matsuzawa S, Salvesen GS, Reed JC, Altieri DC. An IAP-IAP complex inhibits apoptosis. J Biol Chem 2004; 279: 34087-34090.
- Vagnarelli P, Earnshaw WC. Chromosomal passengers: the four-dimensional regulation of mitotic events. Chromosoma 2004; 113: 211-222.
- Grossman D, McNiff JM, Li F, Altieri DC. Expression of the apoptosis inhibitor, survivin, in non-melanoma skin cancer and gene targeting in a keratinocyte cell line. Lab Invest 1999; 79: 1121-1126.
- Thomas J, Liu T, Cotter MA, Florell SR, Robinette K, Hanks AN, Grossman D. Melanocyte expression of survivin promotes development and metastasis of UV-induced melanoma in HGF-transgenic mice. Cancer Res 2007; 67: 5172-5178.
- Bowen AR, Hanks AN, Murphy KJ. Proliferation, apoptosis, and survivin expression in keratinocytic neoplasms and hyperplasias. Am J Dermatopathol 2004; 26: 177-181.
- Bongiovanni L, Colombi I, Fortunato C. Survivin expression in canine epidermis and in canine and human cutaneous squamous cell carcinomas. Vet Dermatol 2009; 20: 369-376.
- Lo Muzio L, Staibano S, Pannone G, Mignogna MD, Mariggio A, Salvatore G, Chieffi P, Tramontano D, De Rosa G, Altieri DC. Expression of the apoptosis inhibitor survivin in aggressive squamous cell carcinoma. Exp Mol Pathol 2001; 70: 249-254.
- Khan Z, Tiwari RP, Mulherkar R, Sah NK, Prasad GB, Shrivastava BR, Bisen PS. Detection of survivin and p53 in human oral cancer: correlation with clinicopathologic findings. Head Neck 2009; 31: 1039-1048.
- Dohi T, Beltrami E, Wall NR. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J Clin Invest 2004; 114: 1117-1127.
- Dohi T, Okada K, Xia F, Wilford CE, Samuel T, Welsh K, Marusawa H, Zou H, Armstrong R, Matsuzawa S, Salvesen GS, Reed JC, Altieri DC. An IAP-IAP complex inhibits apoptosis. J Biol Chem 2004; 279: 34087-34090.
- Roy P, Nigam N, George J. Induction of apoptosis by tea polyphenols mediated through mitochondrial cell death pathway in mouse skin tumors. Cancer Biol Ther 2009; 8: 1281-1287.
- Fan CF, Xu HT, Lin XY, Yu JH, Wang EH. A multiple marker analysis of apoptosis-associated protein expression in non-small cell lung cancer in a Chinese population. Folia Histochem Cytobiol 2011; 49: 231-239.
- McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 2013; 5: 8656.
- Anja P, Simone G, Anna KH, Guido P, Markus W, Ulrich S, Martina R, Rudolf R. Survivin and pAkt as potential prognostic markers in squamous cell carcinoma of the head and neck. Oral Surg Oral Med Oral Pathol Oral Radiol 2014; 117: 733-742.
- Coutinho-Camillo CM, Lourenço SV, Nishimoto IN, Kowalski LP, Soares FA. Caspase expression in oral squamous cell carcinoma. Head Neck 2011; 33: 1191-1198.
- Kim YH, Kim SM, Kim YK, Hong SP, Kim MJ, Myoung H. Evaluation of survivin as a prognostic marker in oral squamous cell carcinoma. J Oral Pathol Med 2010; 39: 368-375.
- Marconi A, Dallaglio K, Lotti R, Vaschieri C, Truzzi F, Fantini F, Pincelli C. Survivin identifies keratinocyte stem cells and is downregulated by anti-beta1 integrin during anoikis. Stem Cells 2007; 25: 149-155.
- Gambichler T, Tigges C, Dith A, Skrygan M, Scola N, Altmeyer P, Kreuter A. Impact of etanercept treatment on ultraviolet B-induced inflammation, cell cycle regulation and DNA damage. Br J Dermatol 2011; 164: 110-115.
- ODonovan N, Crown J, Stunell H, Hill AD, McDermott E, OHiggins N, Duffy MJ. Caspase 3 in breast cancer. Clin Cancer Res 2003; 9: 738-742.
- Tormanen-Napankangas U, Soini Y, Kahlos K, Kinnula V, Paakko P. Expression of caspases-3, -6 and -8 and their relation to apoptosis in non-small cell lung carcinoma. Int J Cancer 2001; 93: 192-198.
- Moon WS, Tarnawski AS. Nuclear translocation of survivin in hepatocellular carcinoma: a key to cancer cell growth? Hum Pathol 2003; 34: 1119-1126.