- Biomedical Research (2016) Volume 27, Issue 3
The role of silver alginate, epithelial growth factor, and platelet rich plasma on wound healing in burn model.
Mucahit Kapci1, Buket Demirci2, Nesibe Kahraman Cetin3, Bekir Daglı1, Ali Duman1, Mucahit Avcil1, Ayhan Akoz1, Kenan Ahmet Turkdogan1*
1Department of Emergency Medicine, Medical Faculty, Adnan Menderes University, Aydin, Turkey
2Department of Medical Pharmacology, Medical Faculty, Adnan Menderes University, Aydin, Turkey
3Department of Pathology, Medical Faculty, Adnan Menderes University, Aydin, Turkey
- *Corresponding Author:
- Kenan Ahmet Turkdogan
Department of Emergency Medicine Medical Faculty
Adnan Menderes University Turkey
Accepted date: April 12, 2016;
Abstract
The aim of this study is to compare the promoting effects of Platelet Rich Plasma (PRP), Silver Alginate (AgS), and intralesional Epidermal Growth Factor (EGF) in burn wound healing through the use of 56 rats equally divided into four groups. The control group was given only saline (SF). Except for AgS, all of the drugs were administered by the intralesional route on the first, third, and fifth days of the experiment. Half of the rats in each group were euthanized on the 14th day and the remaining rats were kept for 21 days to observe the wound healing process. The excised specimens were fixed in a 10% neutral buffered formalin solution for pathological evaluation. In the PRP group, fibroblastic activity and neoangiogenesis decreased between 14 and 21 days while Collagen Deposition (CD) increased. In the EGF group, Fibroblast Proliferation (FP), CD, and neoangiogenesis increased between 14 and 21 days. The correlation was found to be directly proportional to this increase. In the Ag sulfadiazine group, FP and neoangiogenesis did not increase between 14 and 21 days while CD increased slightly. All three drug groups induced wound healing. While Ag sulfadiazine showed uncontrolled wound healing, PRP stimulated more efficient healing in the acute phase and EGF stimulated a more slowly progressing wound healing.
Keywords
Burn, Wound healing, Epithelial growth factor, Silver sulfadiazine, Platelet rich plasma.
Introduction
The healing of wounds has been an important clinical endeavor for centuries. Currently, more wound care products are being produced to treat specific types of wounds. However, the main phases of healing are similar for almost all wounds. Homeostasis, inflammation, proliferation, and remodeling (resolution or maturation) are the main phases of wound healing [1,2]. Because of these phases, many factors can affect tissue repair. As a result, most wound care products are targeted to stimulate one or more of these phases, especially products used for improving delayed tissue repair or stimulating the healing of chronic wounds [3]. The basic events are constructed in the proliferative phase, just behind the inflammatory process. In this phase, dermal growth is started with neoangiogenesis by stimulating the capillary growth with fibroblastic activity and endothelial cell migration [2,4]. Thereafter, collagen formation and deposition provide tissue granulation. The wound bed is composed with fibroblasts by producing major elements of the extracellular matrix, such as collagens, glycosaminoglycans, and proteoglycans [2,4].
Burns are challenging wounds that require critical care and further support for optimizing functional recovery due to tissue loss and exaggerated systemic responses. Furthermore, skin destruction and protein denaturation lead to impaired tissue repair and facilitate microorganismal penetration [5,6]. Thus, wound care products have more importance for these types of wounds [5]. Burns are also investigated in experimental wound care studies due to their complex nature. This study aims to compare the curative and supportive effects of Silver Alginate (AgS), Platelet Rich Plasma (PRP), and intralesional Epidermal Growth Factor (EGF) in experimentally created burn wounds in rats.
Material and Method
Animals
In this study, the weight of the rats was 220-250 g. We applied an iron 1.5 cm in diameter to the shaved back of each rat for 15 seconds to form a 2nd degree burn model. Following the approval of the Animal Ethics Committee (64583101/2015/133), the experimental study was conducted by using 56 three- to four-month-old female rats. During the study period, the rats were kept in individual cages to avoid harming each other, at room temperature (20 ± 2 °C) in 12-hour daylight/dark environments, fed with standard food pellets, and allowed free access to water.
Experimental protocol
In the first day of study, the rats were randomly divided into four main groups of 14: control group, AgS-treated group (Silverdin® 1%, Deva, Istanbul, Turkey), EGF-treated group (Heberprot-P®, Heber Biotec, S.A. La Habana, Cuba), and PRP-treated group. Under the ketamine/xylazine (50 mg/kg+5 mg/kg, intraperitoneally, respectively) anesthesia, the dorsa of the rats were shaved and a preheated hot iron bar was applied for 15 seconds.
After a preheated hot iron bar was applied, the control group was given only saline (SF) on the first, third, and fifth days of the experiment. SF, EGF, and PRP were applied through an intralesional route on the first, third, and fifth days of the experiment. AgS was applied through the transdermal route on the first, third, and fifth days of the experiment. Half of the rats in each group were euthanized on the 14th day under ketamine/ xylazine (50 mg/kg+5 mg/kg, intraperitoneally, respectively) anesthesia and the remaining rats were kept for 21 days to observe the wound healing process (Table 1).
| Groups | n | Treatment of route on 1st day | Treatment of route on 3th day | Treatment of route on 5th day | Specimens taken day |
|---|---|---|---|---|---|
| Control group n=14 | n=7 | intralesional | intralesional | intralesional | 14th day |
| n=7 | intralesional | intralesional | intralesional | 21th day | |
| AgS group n=14 | n=7 | transdermal | transdermal | transdermal | 14th day |
| n=7 | transdermal | transdermal | transdermal | 21th day | |
| EGF group n=14 | n=7 | intralesional | intralesional | intralesional | 14th day |
| n=7 | intralesional | intralesional | intralesional | 21th day | |
| PRP group n=14 | n=7 | intralesional | intralesional | intralesional | 14th day |
| n=7 | intralesional | intralesional | intralesional | 21th day |
Table 1: Design of study.
PRP was freshly prepared as it was previously. Blood samples were withdrawn from the other two rats into anticoagulanttubes with acid citrate dextrose (ACD). When the tubes were centrifuged at low rpm (200/mins, 20°C, 10 mins), three parts were identified. Buffy-coat and platelet-poor plasma, taking place at the top, were re-centrifuged together (300/mins, 20°C, 15 mins) and PRP was obtained.
Histopathologic evaluation
They were entrenched in paraffin wax for examination. Hematoxylin and eosin, Masson's Trichrome, and CD31 were used for tissue staining during examination. CD31 was used to illustrate the neo-angiogenesis better. Masson's Trichrome was used to evaluate the collagen deposition.The Ehrlich/Hunt numerical scale was used for scoring of inflammatory cell infiltration, neo-angiogenesis, FP, and CD. The scores were as follows; 0 for no evidence, 1 for little but scattered, 2 for small amounts and in every field, 3 for large amounts but scattered, and 4 for large amounts and present at every area [7].
Statistical evaluation
Statistical analysis of the data was performed by using Kruskal-Wallis and Chi-square tests. The level of significance was considered as p<0.05. When significant differences were found among groups, pairwise comparisons were performed between groups in order to detect the groups from which the differences originated.
Results
Evaluation of specimens taken on 14th day
There was no significant difference between groups in terms of inflammatory infiltration findings when the specimens were examined. FP was higher in all three drug groups compared to the control group, but the statistical difference was between the control group and the PRP group (p=0.035). The Ag sulfadiazine group had the highest collagen deposition and the difference between the Ag sulfadiazine and the EGF and PRP groups was statistically significant (p=0.035 and p=0.022, respectively). The highest neoangiogenesis was observed in the PRP group and the difference between the PRP and the Ag sulfadiazine groups was statistically significant (p=0.022) (Table 2).
| Control Group | Ags Group | EGF Group | PRP Group 4 | P value | |
|---|---|---|---|---|---|
| 14th day | |||||
| Inflammatoryinfiltration | 1.00 (0.00-2.00) | 1.00(0.00-1.00) | 1.00(0.75-1.25) | 1.00(0.75-2.00) | 0.684 |
| Fibroblasticactivity | 0.00(0.00-1.00) | 1.00(0.00-1.00) | 1.00(0.75-1.25) | 1.00(1.00-2.00)a | 0.035 |
| Collagendeposition | 2.00(1.00-3.00) | 3.00(2.00-3.00) | 2.00(2.00-2.00)b | 2.00(1.75-2.00)b | 0.022 |
| Neo-angiogenesis | 1.00(1.00-2.00) | 1.00(0.00-1.00) | 1.00(0.75-1.25) | 2.00(1.00-2.00)b | 0.022 |
| Epitelial rejenerasyon | 2.00(0.00-2.00) | 1.00(1.00-2.00) | 1.00(1.00-2.00) | 1.00(0.75-1.25) | 0.745 |
| 21th day | |||||
| Inflammatoryinfiltration | 0.00(0.00-1.00) | 1.00(0.00-1.00) | 1.00(0.00-1.00) | 0.00(0.00-1.00) | 0.538 |
| Fibroblasticactivity | 1.00(1.00-2.00)c | 1.00(0.00-1.00)c | 2.00(2.00-2.00) | 1.00(1.00-1.00)c | 0.001 |
| Collagendeposition | 1.00(2.00-2.00)b | 4.00(3.00-4.00) | 2.00(2.00-2.00)b | 2.00(2.00-3.00)b | 0.001 |
| Neo-angiogenesis | 1.00(0.00-1.00)c | 1.00(1.00-1.00)c | 2.00(2.00-2.00) | 1.00(1.00-1.00)c | 0.004 |
| Epitelial rejenerasyon | 2.00(1.00-2.00) | 2.00(2.00-2.00) | 2.00(2.00-2.00) | 2.00(2.00-2.00) | 0.256 |
Abbreviations: Ags; Ag sulfadiazine, EGF: Epidermal Growth Factor, PRP:Platelet Rich Plasma. ap<0.05 vs Control bp<0.05 vs AgS cp<0.01 vs EGF
Table 2: The comparison of median scores (25-75 percentile) of the study groups according to Ehrlich Hunt scale.
Evaluation of specimens taken on 21st day
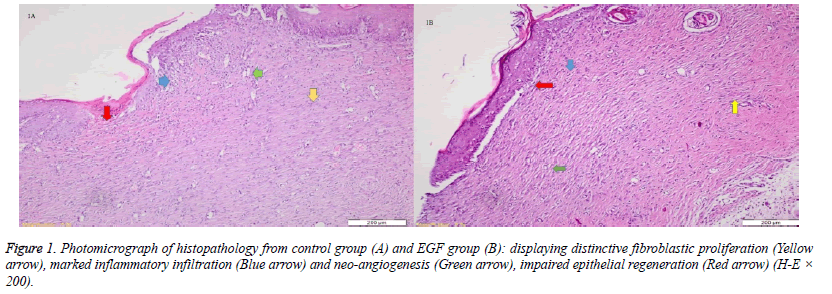
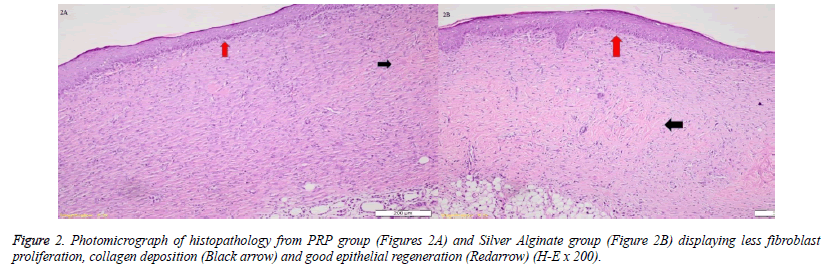
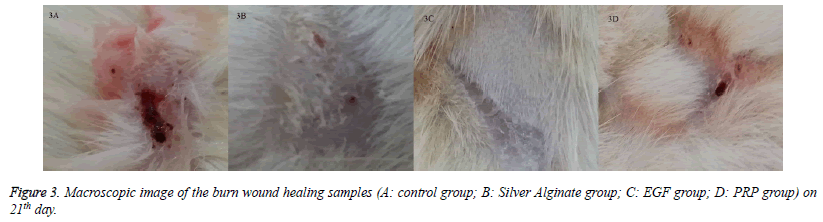
When the specimens were examined, there was no significant difference between the groups in terms of inflammatory infiltration findings. The highest FP was determined in the EGF group and there was a significant difference between the EGF group and the control group, The Ag sulfadiazine group and the PRP group were compared (p=0.026, p=0.004, p=0.001, respectively) (Figures 1A and 1B). The Ag sulfadiazine group had the highest collagen deposition and there was a significant difference when the Ag sulfadiazine group was compared to the control, EGF, and PRP groups (p=0.001, p=0.001, p=0.007, respectively). The highest neoangiogenesis was observed in the EGF group and a significant difference was found when the EGF group was compared to the control, Ag sulfadiazine, and PRP groups (p=0.011, p=0.004, p=0.026, respectively) (Figures 2A and 2B) (Table 2). Macroscopic images of the samples on the 21st day are shown in Figure 3.
Discussion
This is a unique study that compares the promoting effects of intralesional PRP, intralesional EGF, and transdermal AgS. In the PRP group, FP and neoangiogenesis decreased between the 14th and 21st days, while collagen deposition increased. In the EGF group, FP, CD, and neoangiogenesis increased between the 14th and 21st days. A correlation was found to be directly proportional to this increase. In the Ag sulfadiazine group, FP and neoangiogenesis did not increase between the 14th and 21st days, while CD increased slightly more.
In the study conducted by Yenilmez et al., highly damaged and intensely inflamed tissue was observed in the AgS group after treatment for seven days. After 14 days, the damage was still obvious; however, formation of granulation tissue and incomplete reepithelialization was seen. On the 21st day, formation of the granulation tissue and increased collagen fiber formation was evident, as well as incomplete epithelialization [7,8]. In the Ag sulfadiazine group, FP did not increase between the 14th and 21stday, and highly increased CD also indicates an uncontrolled wound healing in our study. The failure of increasing neoangiogenesis also supported this finding.
Platelet-Derived Growth Factor (PDGF) was investigated in 18 patients with 26 lower extremity wounds for a duration of at least eight weeks. Only 78% of the patients were diabetic. Over the 12-week study period, the researchers did not find any improvement in wound healing with the use of PDGF [9]. While this study was limited by a small sample size, its results suggested that the treatment of chronic wounds with PDGF is no better than traditional therapy. In another study, they stated that fistulization did not develop and wound healing was complete in the PRP group for experimentally-prepared pharyngocutaneous wound healing [10]. They showed that PRP was beneficial for acute wound healing in both studies. Results obtained in our study were correlated with the literature. In our study, collagen deposition increased while fibroblastic activity decreased between the14th and 21stdays in the PRP group. This indicates that PRP lost influence on chronic wound healing while stimulating acute wound healing. The decrease of neoangiogenesis in the 21stday also supports this.
In one study, Hardwice et al. found that EGF helped in the healing of acute wounds, but it had a limited effect in the healing of chronic wounds [11]. But, JV Pai-Dhungat et al. said that a shortage of growth factors impairs wound healing, which leads to chronic non-healing wounds. Ischemic foot diabetic ulcer is the most difficult to treat and confers the highest amputation risk. Injecting epidermal growth factor deep into the wound bottom and contours encourages an effective pharmacodynamic response in terms of granulation tissue growth and wound closure [12]. The fact that FP and CD increased between the 14th and 21stdays in the EGF group in this study showed that the wound was healing quite well. An increase of neoangiogenesis also supported this. This also indicated that wound healing was active in both acute and chronic periods. While FP and CD were correlatively higher in wound healing, collagen deposition increased and fibroblastic activity decreased between the 14th and 21stdays in the PRP group in our study. This showed us that PRP's effect lost in chronic wound healing by stimulating acute wound healing. A decrease of neoangiogenesis on the 21st day also supported this. The fact that FP and CD increased between the 14th and 21stdays in the EGF group showed that the wound was healing quite well. An increase of neoangiogenesis also supported this. In the Ag sulfadiazine group, FP did not increase and CD increased more between the 14th and 21stdays, which also indicated an uncontrolled wound healing. The failure of increasing neoangiogenesis also supported this.
Consequently, all three drug groups induced wound healing. While Ag sulfadiazine showed uncontrolled wound healing, PRP stimulated a more active healing in the acute phase, and EGF stimulated a slowly progressing wound healing. In the chronic period, neoangiogenesis increased only in the EGF group. We think that EGF may provide an additional benefit when applied to advanced burns due to this effect.
Limitation
The major limitation of our study was that we had no fourth week (28th day) group. This situation makes it hard to reveal the group demonstrating the best organization in complete wound healing. However, this pilot study may lead to further studies that will examine this issue in the future. The second limitation was that the study was designed on an animal model. Thus, our results need to be verified on humans. The third limitation was that the animals couldn’t be treated on the seventh day. These limitations may clarify whether collagen deposition will occur in the AgS group when compared with other groups.
Acknowledgement
We wish to thank Hasbiotech and Dr. Oguz Karahan for gifting us with the standardized EGF and Prof. Dr. Zahit Bolaman and Laboratory technician Atilla Karada? for preparing PRP.
References
- Mathieu D, Linke J-C, Wattel F. Non-healing wounds. In: Handbook on hyperbaric medicine, Mathieu DE, editor. Netherlands: Springer, 2006: pp. 401-427
- Guo S, Di-Pietro LA. Factors Affecting Wound Healing. J Dental Res 2010;89:219-229.
- Murphy PS, Evans GR. Advances in wound healing: a review of current wound healing products. Plast Surg Int 2012;2012:190436.
- Campos AC, Groth AK, Branco AB. Assessment and nutritional aspects of wound healing. Curr Opin Clin Nutr Metab Care 2008; 11:281-288.
- Rowan MP, Cancio LC, Elster EA, Burmeister DM, Rose LF, Natesan S, Chan RK, Christy RJ, Chung KK. Burn wound healing and treatment: review and advancements. Crit Care 2015;19: 243.
- Nisanci M, Eski M, Sahin I, Ilgan S, Isik S. Saving the zone of stasis in burns with activated protein C: an experimental study in rats. Burns 2010;36:397-402.
- Ehrlich HP, Tarver H, Hunt TK. Effects of vitamin A and glucocorticoids upon inflammation and collagen synthesis. Ann Surg 1973;177:222-227.
- Yenilmez E, Basaran E, Arslan R, Berkman MS, Güven UM, Baycu C, Yazan Y. Chitosan gel formulations containing egg yolk oil and epidermal growth factor for dermal burn treatment. Pharmazie 2015;70:67-73.
- Krupski WC, Reilly LM, Perez S, Moss KM, Crombleholme PA, Rapp JH. A prospective randomized trial of autologous platelet-derived wound healing factors for treatment of chronic nonhealing wounds: a preliminary report. J Vasc Surg 1991;14:526-532.
- Eryilmaz A, Demirci B, Gunel C, Doger FK, Yukselen O, Omurlu IK, Basal Y, Agdas F, Basak S. Can tissue adhesives and platelet-rich plasma prevent pharyngocutaneous fistula formation? Auris Nasus Larynx 2016;43:62-67.
- Hardwicke J, Schmaljohann D, Boyce D, Thomas D. Epidermal growth factor therapy and wound healing--past, present and future perspectives. Surgeon 2008;6:172-177.
- Pai-Dhungat JV, Parikh F. Albert Szent-Gyorgyi: Discoverer of Vitamin C India J Associa Physici 2015; 63:142-143.