Research Article - Biomedical Research (2017) Volume 28, Issue 9
The role of miR-21 and its predicted target gene, PTEN, in the development of ventilator associated pneumonia
Zhen Wang1, Yisheng Zheng2, Zhenjian Fang3 and Yuan Zhang4*1Department of Respiratory Medicine, Ningbo No. 7 Hospital, Ningbo, PR China
2Department of Respiratory and Critical Care Medicine, Fuzong Clinical College of Fujian Medical University, Fuzhou General Hospital, Fuzhou, PR China
3Department of Respiratory Medicine, Fuding Hospital, Fuding, PR China
4Department of Respiratory Medicine, Ningbo Zhenhai Longsai Hospital, Ningbo, PR China
- *Corresponding Author:
- Yuan Zhang
Department of Respiratory Medicine
Ningbo Zhenhai Longsai Hospital, PR China
Accepted on January 31, 2017
Abstract
Aims: This study is to analyse the role and mechanism of PTEN in patients with Ventilator-Associated Pneumonia (VAP).
Methods: Alveolar Macrophages (AMs), serum and peripheral blood monocytes were collected from 69 patients with hospital-acquired VAP during the treatment of lung trauma between June, 2012 and July, 2015. Normal samples from 53 healthy people were collected as control group. The levels of PTEN mRNA expression in these samples were examined by quantitative Real-time PCR, while PTEN protein levels were analysed by Western Blot and ELISA. MiRNAs that regulate PTEN expression was predicted by bioinformatics analysis, and the expression of predicted miRNA was measured by quantitative Real-time PCR.
Results: Compared with healthy controls, PTEN was significantly downregulated at both mRNA and protein levels in AMs, serum and peripheral blood monocytes from patients with VAP (P<0.05). MiR-21 was predicted to bind to the 3’UTR of PTEN mRNA, and miR-21 expression was significantly upregulated in samples from SAP patients (P<0.05).
Conclusion: The downregulation of PTEN expression in VAP patients may be directly regulated by the upregulation of miR-21. MiR-21 might affect the function of macrophages by regulating the expression of PTEN, and therefore contribute to the development of VAP.
Keywords
miRNA-21, Protein tyrosine phosphatase gene (PTEN), Ventilator associated pneumonia (VAP), Lung trauma.
Introduction
Ventilator-Associated Pneumonia (VAP) is the most common hospital-acquired infection, and the occurrence of VAP is higher than Central Line-associated Bloodstream Infections (CRBSI) in Intensive Care Units (ICUs) [1]. Epidemiological survey in foreign countries showed that the occurrence of VAP is 10-70% and the mortality rate of VAP is 16-94% [2-4]. In China, the occurrence of VAP is 7-90% and the mortality rate is 50-69% [2]. Therefore, VAP caused great suffering and economic loss of the patients.
Human immunity is closely associated with VAP, in that decrease of body resistance caused by pathogens or increase of cell-mediated allergy may lead to the clinical onset of VAP [5]. It is acknowledged that monocytes-macrophages participate in the development of pneumonia. Blood monocytes exert the function of phagocytosis and give rise to lung macrophages through multiple steps [6,7]. Activated monocytes and macrophages produce a range of cytotoxins, interferons and interleukins, which participate in the defense mechanisms of body, as well as growth factors that promoting the growth of endothelial cells and smooth muscle cell [8,9]. In addition, monocytes tend to divide around inflammation and surround foreign substances [8,9]. Taken together, we speculated that during the development of VAP, a serial of physiological changes occur in monocytes-macrophages and the cytokines produced by these cells are changed, which result in the outcome of VAP.
Protein Tyrosine Phosphatase Gene (PTEN) is an important gene in human and it has been wildly studied in tumors. PTEN plays a key role in cell apoptosis, cell cycle and cell migration, and inactivation of this gene by mutation is associated with the development of multiple malignant tumors [10]. PTEN expression is also closely related to pulmonary infection. It has been reported that PTEN was involved in the immunity and inflammatory after pulmonary infection [11-13]. However, little is known about the expression profile of PTEN, as well as regulators upstream of PTEN, in the development of VAP.
The main objective of this study was to elucidate the regulation of PTEN in VAP and its mechanism, focusing on its upstream regulators. We investigated the expression of PTEN mRNA and protein by Real-time PCR, Western blot and ELISA in Alveolar Macrophages (AMs), serum and peripheral blood monocytes from patients with VAP. We show that PTEN is a promising target of miR-21 by bioinformatics prediction and its expression is negative correlated with that of PTEN.
Materials and Methods
Patients
A total of 69 patients with hospital-acquired Ventilator Associated Pneumonia (VAP) during the treatment of lung trauma and 53 healthy controls from the Laiwu people’s hospital between June, 2012 and July, 2015 were enrolled in the study. Healthy controls were no-smoking volunteers that confirmed by fiberoptic bronchoscopy, chest X-ray and pulmonary function test. Data of patient characteristics is shown in Table 1. Prior written and informed consent were obtained from every patient and the study was approved by the ethics review board of Laiwu people’s hospital.
| n | Male | Female | Age (Range) | Age (Median) | |
|---|---|---|---|---|---|
| Controls | 69 | 35 | 18 | 22-61 | 46 |
| Patients | 53 | 47 | 22 | 25-72 | 51 |
Table 1. Sample characterization.
Diagnosis of VAP was in accordance of the guidelines for the management of adults with hospital-acquired, ventilatorassociated, and healthcare-associated pneumonia by American thoracic society and the infectious diseases society of America in 2005 [1]. In general, two criteria should be met. First, the presence of a new or progressive radiographic infiltrate. Second, at least two of three clinical features including fever greater than 38˚C or hypothermia lower than 36˚C, leukocytosis (PBMC>10 × 109/L) or leucopenia (PBMC<4 × 109/L) and purulent secretions in trachea or bronchus. Patients with diseases such as pulmonary oedema, tuberculosis, Acute Respiratory Distress Syndrome (ARDS) and pulmonary embolism were not included.
Patients with complications in organs such as heart, liver and kidney, diabetes, tumors and autoimmune diseases were excluded as confirmed by pathobiologist from Laiwu people’s hospital.
Sample collection
Three types of samples were collected for further study. Bronchoalveolar Lavage (BAL) and Alveolar Macrophages (AMs) were collected during fiberoptic bronchoscopy. In general, after local anesthesia of the respiratory tract, the BF-1T260 fiberscope (Olympus optical Co., Ltd., Tokyo, Japan) was inserted, placed into the orifice of segmental or subsegmental bronchus, and 4-6 volumes of 20 ml of normal saline solution were instilled and retrieved immediately by gentle aspiration with the recovery rate of 40%-60%. Recovered liquid was filtrated, centrifuged and the cells were suspended into 1-3 × 105 cells/ml. Cell viability was 90% as determined by heliogen blue staining. The cell suspend were pelleted onto glass slides and incubated for 60 min at 37°C. Cells failed to adhere were washed off by 0.5 mol/L PBS and cells adhering to slides were macrophages. AMs were stored at -20°C until analysis.
Serum was collected from sterile peripheral blood from subjects. In general, 10-15 ml of peripheral blood were placed in 4°C for 1-2 h and serum in the upper layer were collected after being centrifuged for 10 min at 400 Xg. Serum were aliquoted and sored at -70°C.
Peripheral blood monocytes were also isolated from peripheral blood. Anticoagulant-treated blood was dilute with an equal volume of Iscove's Modified Dulbecco's Medium (IMDM, Invitrogen, Waltham, MA, USA). Eight ml diluted blood were layered over 5 ml of Ficoll-Hypaque in a sterile 15 ml conical tube by dribbling slowly down the side of the tube using a pipet. After centrifuging at 400 Xg for 30 minutes, white cell layer containing the mononuclear cells were in the middle layer. The white cells were removed and placed in a sterile 15 ml polypropylene tube. Cells were then washed twice with 5 volumes of D-Hank’s and centrifuged at 300 Xg for 10 minutes. Cells were resuspended into 1 × 106 cells/ml, and 3 × 106 cells were inoculated into 9 cm2 plates followed by culture for 1-2 h at 37°C. Adherent cells were peripheral blood monocytes.
RNA isolation and quantitative real-time PCR
Total RNA was extracted by Trizol Reagent (Yusheng Biotech, Shanghai, China). MiRNAs were extracted by miRcute miRNA isolation kit (Tiangen, Beijing, China) form cells and by miRNeasy Serum/Plasma Kit (Jianlun Biotech, Guangzhou, China) form serum. Following gel electrophoresis verification of RNA integrity and quantification using UV spectrophotometer, mRNA was reverse transcribed by TIANScript IIcDNA kit (Tiangen, Beijing, China), and miRNA was reverse transcribed by miRcute miRNA cDNAkit (Tiangen, Beijing, China).
Quantitative real-time PCR was performed using a miRcute miRNA qPCR detection kit (Tiangen, Beijing, China) for miRNAs and SuperReal PreMix (SYBR Green) (Tiangen, Beijing, China) for mRNA on IQ-5 (Bio-Rad, Hercules, California, USA). For mRNA quantification, the reaction mixture was incubated for 1 cycle at 95°C for 5 min, followed by 40 cycles at 95°C for 30 s, 55°C for 30 s, and 72°C for 60 s. For miRNA, the reaction mixture was incubated for 1 cycle at 95°C for 5 min, followed by 40 cycles at 95°C for 10 s, 62°C for 20 s, and 72°C for 30 s. Primers used for detection was shown in Table 2. The relative expression levels were evaluated using the 2−ΔΔCt method, and the expression of β- actin or U6 snRNA was used as internal control for mRNA and miRNA, respectively.
| Primers | Primer sequence (5’-3’) |
|---|---|
| PTEN_Forward | 5'-TTGAAGACCATAACCCACCACAG -3' |
| PTEN_Backward | 5'-CATTACACCAGTTCGTCCCTTTC -3' |
| β-actin_Forward | 5'-AAGATGACCCAGATCATGTTTGAGACC -3' |
| β-actin_Backward | 5'-GCCAGGTCCAGACGCAGGAT -3' |
| miR-21_Forward | 5’-GCCCGCTAGCTTATCAGACTGATG -3’ |
| miR-21_Backward | 5’-GTGCAGGGTCCGAGGT -3’ |
| U6_Forward | 5’-CTCGCTTCGGCAGCACA -3’ |
| U6_Backward | 5’-AACGCTTCACGAATTTGCGT -3’ |
Table 2. Primers used for real-time PCR.
Western blot
Proteins were extracted by protein extract kit (BestBio, Shanghai, China) and determined by BCA assay kit (Zhongruiketai, Beijing, China). A total of 20 ug proteins were separated on 10 % SDS-Page and transferred to Polyvinylidene Difluoride membranes (PVDF). After blocking with 5% skimmed milk, the membranes were probed with the following antibodies: rabbit anti-PTEN polyclonal antibody (1: 500, ab32199, Abcam, Cambridge, UK) and rabbitanti-β-actin monoclonal antibody (1:5000, ab6276, Abcam). For detection, goat anti-rabbit (1:3000, ab6721, Abcam) secondary antibodies conjugated to HRP (Abcam) were used. Signal detection was performed using chemiluminescence reaction (ECL) (ab65623, Abcam, GA, USA). The acquired images were analysed by Image lab 3.0 (Bio-Rad, Hercules, California, USA) and the relative protein expression was expressed as the densitometric value ratio of PTEN band to β-actin band.
ELISA
PTEN protein levels in serum were measured by PTEN ELISA kit (Bolingkewei, Beijing, China). In brief, 1:4 dilution of serum samples and eight serial dilutions of standard substrate at the volume of 50 ul were incubated overnight at 4°C. After secondary antibody incubation followed by incubation with Horseradish-Peroxidase (HRP) for 1 h, the chromogenic substrate solution was added and the reaction was stopped with H2SO4 and read at 450 nm.
Bioinformatics analysis
To identify the miRNAs that may regulate the expression of PTEN, five bioinformatics software (miRanda, TargetSean, PicTar, MiRanda and BibiServ)were used for a reliable prediction of miRNAs that may target PTEN mRNA.
Statistical analysis
Data analysis was carried out using the trial version of SPSS 18.0 (IBM Corp, Chicago, IL, USA) and expressed as mean ± SD. Normal test was performed. Differences between groups were evaluated for significance using one-way ANOVA followed by post hoc test. LSD or SNK methods were used when variances were equal, and Tamhane's T2 or Dunnett's T3 methods were used when variances were unequal. P<0.05 was considered as significant.
Results
PTEN mRNA expression was downregulated in patients with VAP
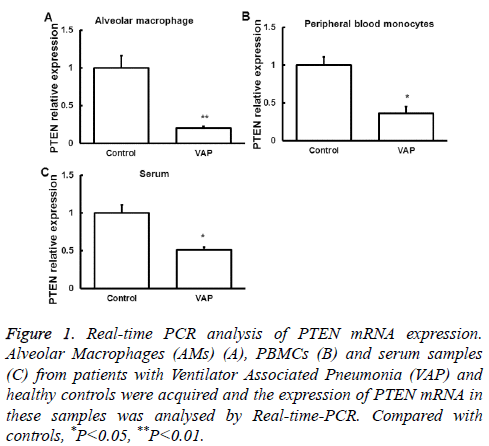
We first examined the expression of PTEN mRNA in Alveolar Macrophages (AMs), serum and peripheral blood monocytes from patients with VAP by quantitative Real-time PCR. Samples from normal subjects were used as control. Compared with that in normal controls, PTEN mRNA expression in the three samples was significantly downregulated (P<0.05, Figure 1), suggesting a regulatory role of PTEN in the development of SAP.
Figure 1: Real-time PCR analysis of PTEN mRNA expression. Alveolar Macrophages (AMs) (A), PBMCs (B) and serum samples (C) from patients with Ventilator Associated Pneumonia (VAP) and healthy controls were acquired and the expression of PTEN mRNA in these samples was analysed by Real-time-PCR. Compared with controls, *P<0.05, **P<0.01.
PTEN protein expression was downregulated in patients with VAP
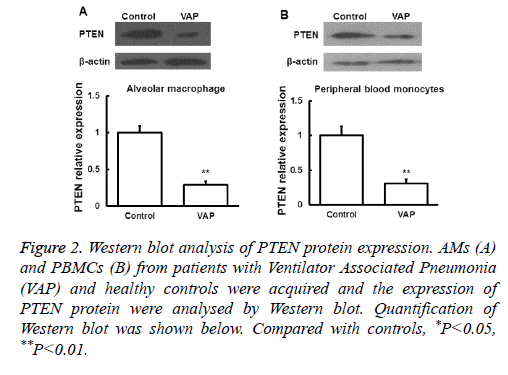
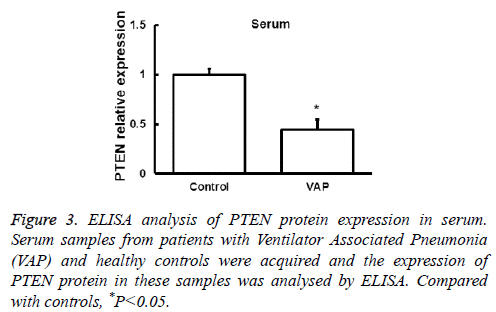
Next the expression of PTEN at the protein level was analysed by Western blot in AMs and peripheral blood monocytes. PTEN protein level was also downregulated in these cells from VAP patients compared with healthy controls (P<0.05, Figure 2). We further investigated the expression of PTEN protein in serum by ELISA and found that the changes of PTEN protein expression was in consistent with its mRNA expression (P<0.05, Figure 3). These results showed that in AMs, serum and peripheral blood monocytes from patients with VAP, PTEN was downregulated at both mRNA and protein level, which indicating that downregulated PTEN expression may regulate VAP.
Figure 2: Western blot analysis of PTEN protein expression. AMs (A) and PBMCs (B) from patients with Ventilator Associated Pneumonia (VAP) and healthy controls were acquired and the expression of PTEN protein were analysed by Western blot. Quantification of Western blot was shown below. Compared with controls, *P<0.05, **P<0.01.
MiR-21 may regulate the expression of PTEN
To investigate the possible mechanism that regulating PTEN expression, miRNAs that may binds to the 3’ untranslated region (3’ UTR) of PTEN was predicted using bioinformatics methods. MiR-21 was predicted to directly bind to the 3’ UTR of PTEN (Figure 4).
MiR-21 was upregulated in patients with VAP
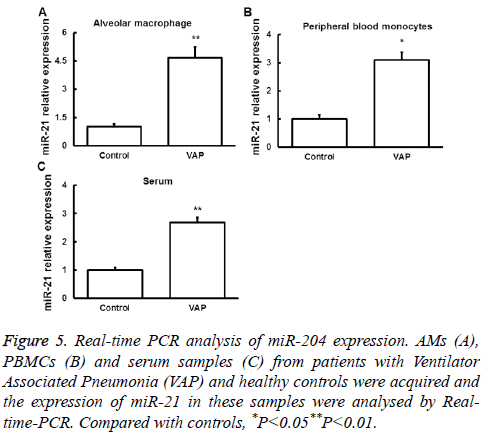
Real-time PCR was used to detect miR-21 expression in AMs, serum and peripheral blood monocytes from patients with VAP and normal controls. We found that miR-21 expression was significantly upregulated in samples from VAP patients (Figure 5). These results indicated that miR-21 may contribute to the regulation of SAP by targeting PTEN mRNA and the resulted downregulation of PTEN protein.
Figure 5: Real-time PCR analysis of miR-204 expression. AMs (A), PBMCs (B) and serum samples (C) from patients with Ventilator Associated Pneumonia (VAP) and healthy controls were acquired and the expression of miR-21 in these samples were analysed by Realtime- PCR. Compared with controls, *P<0.05**P<0.01.
Discussion
VAP, as a common hospital-acquired infection, is a kind of lung infection that develops in people who are on breathing machines [1]. The mortality rate of VAP is 16-94% in the Westerns [2-4] and 50-69% in China [2], indicating that VAP significantly increases risk of mortality. Therefore effective prevention and treatment of this disease is critical. It has been suggested that PTEN is involved in the immune and inflammatory process of pulmonary infection [9-11]. However, little is known about the role of PTEN and its upstream regulatory factors in the pathogenesis of VAP. In the present study, to investigate the mechanism of the development of VAP, expression of PTEN at mRNA and protein level as well as its possible upstream regulator miR-21 in AMs, serum and peripheral blood monocytes from patients with VAP was studied.
The major obstacle in the prevention and treatment of VAP is the difficulty in early diagnosis due to lack of disease-specific early diagnosis markers, leading to high morbidity and mortality. Therefore, establishing a rapid, sensitive and effective diagnosis method based on VAP-specific biological marker is critical for this disease. Factors related to immunity as biomarkers or therapeutic targets have always been the important research direction since human immunity plays important role in the development of VAP [14].
Among cells involved in immune reposes, including macrophages, T lymphocytes and Natural Killer cells (NKs), macrophages was reported to function in VAP [14]. Monocytes-macrophages originate from bone marrow stem cells, and they are then released into the peripheral blood before giving rise to a variety of tissue-resident macrophages throughout the body. As an important part of immunity system, these cells provide the first line of defense against foreign pathogens before the initiation of adaptive immunity [15].Pathogens induce the activation of monocytes-macrophages, and the release of TNF-α and IL-6 into blood [16].
The biological function and the underlying mechanism of PTEN gene is not fully understood so far. Three main pathways downstream of PTEN has been identified, namely phosphatidylinositol 3, 4, 5-trisphosphate (PIP3) pathway, Mitogen Activated Protein Kinase (MAPK) pathway and Focal Adhesion Kinase (FAK) pathway. The synergy of these pathways positively regulates cell cycle, induce cell apoptosis, as well as inhibit cell physiology such as cell growth and migration [17-22]. Nowadays studies on PTEN gene usually focused on tumors including glioma, prostate cancer and breast cancer [23-25], but only a few literatures reported its role in the development of VAP. Here we studied how PTEN gene regulates the function of monocytes-macrophages and its underlying mechanism. During the development of VAP, monocytes-macrophages are activated by infection of pathogens to generate abundant immune responses. We found that PTEN mRNA as well as protein was downregulated in peripheral blood monocytes, AMs and serum from VAP patients, indicating that downregulation of PTEN may regulate the apoptosis of monocytes-macrophages. It is speculated that decreased PTEN expression reduced the apoptosis of monocytes-macrophages, keeping more activated cells for the phagocytosis of pathogens.
Regulation of the transcription and expression of mRNA is a multi-factors involved complicated process. We focused on miRNAs, a class of recently discovered single-strand RNAs at the length of 21-23 nt that repress the translation or decrease the stability of mRNAs by incomplete complementary binding to the 3’ UTR of target mRNAs [26,27], when investigated the upstream regulators of PTEN. Circulating miRNAs was released by normal cells or damaged cells into blood, where they participate in cell signal transduction and the conversion of genetic information [28,29]. MiRNAs are in a very stable state in the peripheral blood, and they are hard to be degraded by RNase due to their package mechanisms [30]. In addition, concentration of miRNAs is relatively high in both plasma and serum, reaching the detection concentration as a biomarker [31]. By bioinformatics prediction, we found that miR-21 is a possible regulator of PTEN by directly binding to its 3’ UTR as the seed sequence of miR-21 (position 2-8) is completely complementary to 14-20 of the PTEN 3’ UTR sequence. Many researches also reported the close relationship between miR-21 and PTEN, and most of them were focused on tumors, such as colon cancer [32,33], bladder cancer [34], chronic myelogenous leukemia [35], cervical cancer [36] and intrahepatic cholangiocarcinoma [37]. Other studies revealed the regulation of miR-21 on PTEN in the context of lung function, including pulmonary artery vascular smooth muscle cells [38], and non-small cell lung cancer [39]. Herein we also observed the upregulation of miR-21 in all the three samples from VAP patients, which is opposite to the expression of PTEN. These results collectively suggested that miR-21 may negatively regulate PTEN expression, which is in accordance with our expectation and previous studies. Accordingly, upregulated miR-21 in VAP patients results in reduced PTEN levels, which further decreases the apoptosis of monocytesmacrophages and promotes the development of VAP.
In summary, our research demonstrated that in the development of VAP, miR-21 is upregulated to reduce the expression of PTEN, and further regulate the function of monocytes-macrophages. Because of the internal regulatory relationship in miR-21-PTEN-immune system, studies on the regulation of miR-21 on PTEN make miR-21 a candidate for the treatment of many immune-related diseases, such as tumors, rheumatoid arthritis and lupus erythematosus. Further studies are needed to investigate the specific role of miR-21 in VAP at cell, animal and clinical levels.
Acknowledgements
We thank Professor Chunting Wang (ICU, Hospital of Shandong Province) for helpful discussion and excellent technical assistance.
Disclosures
All authors declare no financial competing interests. All authors declare no non-financial competing interests.
References
- American Thoracic Society and Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J RespirCrit Care Med 2005; 171: 388-416.
- Arabi Y, Al-Shirawi N, Memish Z, Anzueto A. Ventilator-associated pneumonia in adults in developing countries: a systematic review. Int J Infect Dis 2008; 12: 505-512.
- Valencia M, Torres A. Ventilator-associated pneumonia. CurrOpinCrit Care 2009; 15: 30-35.
- Rotstein C, Evans G, Born A, Grossman R, Light RB, Magder S, McTaggart B, Weiss K, Zhanel GG. Clinical practice guidelines for hospital-acquired pneumonia and ventilator-associated pneumonia in adults. Can J Infect Dis Med Microbiol 2008; 19: 19-53.
- Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD, Kreisel D, Krupnick AS, Srivastava A, Swanson PE, Green JM, Hotchkiss RS. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011; 306: 2594-2605.
- Perez-Rial S, del Puerto-Nevado L, Terron-Exposito R, Giron-Martinez A, Gonzalez-Mangado N, Peces-Barba G. Role of recently migrated monocytes in cigarette smoke-induced lung inflammation in different strain of mice. PLoS One 2013; 8: e72975.
- van Furth R. Origin and kinetics of monocytes and macrophages. SeminHematol 1970; 7: 125-141.
- Junqueira-Kipnis AP, Kipnis A, Jamieson A, Juarrero MG, Diefenbach A, Raulet DH, Turner J, Orme IM. NK cells respond to pulmonary infection with Mycobacterium tuberculosis, but play a minimal role in protection. J Immunol 2003; 171: 6039-6045.
- Sugawara I, Yamada H, Mizuno S, Li CY, Nakayama T. Mycobacterial infection in natural killer T cell knockout mice. Tuberculosis (Edinb) 2002; 82: 97-104.
- Squarize CH, Castilho RM, Santos Pinto D. Immunohistochemical evidence of PTEN in oral squamous cell carcinoma and its correlation with the histological malignancy grading system. J Oral Pathol Med 2002; 31: 379-384.
- Li Y, Jia Y, Pichavant M, Loison F, Sarraj B, Kasorn A, You J, Robson BE, Umetsu DT, Mizgerd JP, Ye K, Luo HR. Targeted deletion of tumor suppressor PTEN augments neutrophil function and enhances host defense in neutropenia-associated pneumonia. Blood 2009; 113: 4930-4941.
- Schabbauer G, Matt U, Gunzl P, Warszawska J, Furtner T, Hainzl E, Elbau I, Mesteri I, Doninger B, Binder BR, Knapp S. Myeloid PTEN promotes inflammation but impairs bactericidal activities during murine pneumococcal pneumonia. J Immunol 2010; 185: 468-476.
- Hubbard LL, Wilke CA, White ES, Moore BB. PTEN limits alveolar macrophage function against Pseudomonas aeruginosa after bone marrow transplantation. Am J Respir Cell MolBiol 2011; 45: 1050-1058.
- Christaki E. Host immune response in sepsis due to ventilator-associated pneumonia: how is it different? Crit Care 2009; 13: 1009.
- Hume DA. The many alternative faces of macrophage activation. Front Immunol 2015; 6: 370.
- Shitrit D, Izbicki G, Bar-Gil Shitrit A, Raz M, Sulkes J, Kramer MR. Role of soluble interleukin-2 receptor levels in patients with latent tuberculosis. Lung 2006; 184: 21-24.
- McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, Navolanic PM, Terrian DM, Franklin RA, DAssoro AB, Salisbury JL, Mazzarino MC, Stivala F, Libra M. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul 2006; 46: 249-279.
- Tamura M, Gu J, Takino T, Yamada KM. Tumor suppressor PTEN inhibition of cell invasion, migration, and growth: differential involvement of focal adhesion kinase and p130Cas. Cancer Res 1999; 59: 442-449.
- Davies MA, Koul D, Dhesi H, Berman R, McDonnell TJ, McConkey D, Yung WK, Steck PA. Regulation of Akt/PKB activity, cellular growth, and apoptosis in prostate carcinoma cells by MMAC/PTEN. Cancer Res 1999; 59: 2551-2556.
- Stiles B, Gilman V, Khanzenzon N, Lesche R, Li A. Essential role of AKT-1/protein kinase B alpha in PTEN-controlled tumorigenesis. Mol Cell Biol 2002; 22: 3842-3851.
- Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell 2000; 100: 387-390.
- Li DM, Sun H. PTEN/MMAC1/TEP1 suppresses the tumorigenicity and induces G1 cell cycle arrest in human glioblastoma cells. ProcNatlAcadSci USA 1998; 95: 15406-15411.
- Duerr EM, Rollbrocker B, Hayashi Y, Peters N, Meyer-Puttlitz B. PTEN mutations in gliomas and glioneuronal tumors. Oncogene 1998; 16: 2259-2264.
- Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Baak JP. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst 2000; 92: 924-930.
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997; 275: 1943-1947.
- Lewis BP, Burge CB,Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005; 120: 15-20.
- Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet 2007; 8: 93-103.
- Nakamura T, Canaani E, Croce CM. Oncogenic All1 fusion proteins target Drosha-mediated microRNA processing. ProcNatlAcadSci USA 2007; 104: 10980-10985.
- Guo L. Advances in micro RNA and apoptosis. ProgPhysiolSci 2007; 38: 331-334.
- Li X, Yu Z, Li Y, Liu S, Gao C, Hou X, Yao R, Cui L. The tumor suppressor miR-124 inhibits cell proliferation by targeting STAT3 and functions as a prognostic marker for postoperative NSCLC patients. Int J Oncol 2015; 46: 798-808.
- Lv ZC, Fan YS, Chen HB, Zhao DW. Investigation of microRNA-155 as a serum diagnostic and prognostic biomarker for colorectal cancer. Tumour Biol 2015; 36: 1619-1625.
- Tao YJ, Li YJ, Zheng W, Zhao JJ, Guo MM, Zhou Y, Qin NL, Zheng J, Xu L. Antisense oligonucleotides against microRNA-21 reduced the proliferation and migration of human colon carcinoma cells. Cancer Cell Int 2015; 15: 77.
- Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML,Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Molecular Cell 2010; 39:493-506.
- Lei M, Xie W, Sun E, Sun Y, Tian D. microRNA-21 regulates cell proliferation and migration and cross talk with PTEN and p53 in bladder cancer. DNA Cell Biol 2015; 34: 626-632.
- Taverna S, Giallombardo M, Pucci M, Flugy A, Manno M, Raccosta S, Rolfo C, De Leo G, Alessandro R. Curcumin inhibits in vitro and in vivo chronic myelogenousleukemia cells growth: a possible role for exosomal disposal of miR-21. Oncotarget 2015; 6: 21918-21933.
- Shishodia G, Shukla S, Srivastava Y, Masaldan S, Mehta S, Bhambhani S, Sharma S, Mehrotra R, Das BC,Bharti AC. Alterations in microRNAs miR-21 and let-7a correlate with aberrant STAT3 signalling and downstream effects during cervical carcinogenesis. Mol Cancer 2015; 14: 116.
- Wang LJ, He CC, Sui X, Cai MJ, Zhou CY, Ma JL, Wu L, Wang H, Han SX, Zhu Q. MiR-21 promotes intrahepatic cholangiocarcinoma proliferation and growth in vitro and in vivo by targeting PTPN14 and PTEN. Oncotarget 2015; 6: 5932-5946.
- Green DE, Murphy TC, Kang BY, Searles CD, Hart CM. PPARgammaligands attenuate hypoxia-induced proliferation in human pulmonary artery smooth muscle cells through modulation of microRNA-21. PLoS One 2015; 10: e0133391.
- Yang Z, Fang S, Di Y, Ying W, Tan Y,Gu W. Modulation of NF-kappaB/miR-21/PTEN pathway sensitizes non-small cell lung cancer to cisplatin. PLoS One 2015; 10: e0121547.