Review Article - Archives of General Internal Medicine (2018) Volume 2, Issue 2
The Role of Blood-Brain Barrier Damage in the Pathogenesis of Contrast-Induced Encephalopathy.
Guilian Zhang*, Heying Wang, Tao Li, Jiao Liu, Lili Zhao, Man Sun and Yating Jian
Department of Neurology, The Second Affiliated Hospital, Medical School of Xi’an Jiaotong University, Xi’an 710004, P.R. China
- *Corresponding Author:
- Guilian Zhang
Department of Neurology
The Second Affiliated Hospital
Medical School of Xi’an Jiaotong University
Xi’an 710004, P.R. China
E-mail: zhgl_2006@126.com
Accepted on March 30, 2018
Citation: Zhang G, Wang H, Li T, et al. The role of blood-brain barrier damage in the pathogenesis of contrast-induced encephalopathy. Arch Gen Intern Med. 2018;2(2):34-40.
Abstract
Contrast media are widely used in diagnostic and interventional angiographic procedures. While they are generally regarded as safe and effective, some risks are associated with contrast media. Contrast-induced encephalopathy is a rare adverse reaction to contrast media, and a number of cases have been reported in various countries. The condition may manifest with psychiatric symptoms, cortical blindness, epilepsy or focal neurological deficits, and can be difficult to distinguish from other cerebrovascular complications. Therefore, better understanding of the condition would be valuable for determining the most appropriate treatment. A range of evidence suggests that blood-brain barrier damage is involved in the pathogenesis of the disorder. Here we briefly review the properties of iodinated contrast media and corresponding changes of the blood-brain barrier.Keywords
Contrast media, Blood-brain barrier, Contrast-induced encephalopathy, Pathogenesis.
Introduction
The first use of iodine contrast media (CM) in clinical dates back to the 1920s [1]. Since 1950s, iodinated contrast media have been the most widely used pharmaceuticals for diagnostic and interventional angiographic procedures. With the development of medical technology and the widespread application of them, adverse reactions to iodinated CM have attracted substantial clinical attention. These adverse effects range from mild discomfort to life-threatening events including specific responses, shock, congestive heart failure, arrhythmia, acute renal failure and neurotoxicity, and have been reported in a number of studies [2,3]. CM does not generally cross the blood-brain barrier (BBB) to enter the central nervous system. However, damage to the BBB can increase its permeability, allowing CM to permeate into the brain and exert toxic effects 3. In 1970, Fischer-Williams et al. [4] reported the first case of a patient exhibiting transient cortical blindness after diatrizoate methylglucamine was administered for coronary angiography. This condition was initially regarded as an adverse reaction to CM, and later became termed contrast-induced encephalopathy (CIE). Although the pathogenesis of CIE has not yet been fully elucidated, changes in BBB permeability are thought to be involved [3,5,6]. The current review provides an overview of the role of BBB damage in the pathogenesis of CIE, briefly describing the properties of iodinated CM and the corresponding changes in the BBB.
Classification and properties of iodinated CM
Iodinated CM is typically divided into high-, low- or iso-osmolal types, according to the level of osmotic pressure. In addition, they are classified according to their ionicity (ionic and non-ionic types) and the number of benzene rings (monomers and dimers) [7,8]. To date, three generations of CM have been developed [9]. First-generation (e.g., diatrizoate methylglucamine, iothalamate, metrizoate) or high-osmolar CM (HOCM) are ionic monomers with an osmotic pressure of approximately 2000 mOsm/L, which is substantially higher than plasma. The second generation of CM exhibit increased hydrophilicity, including not only ionic dimers (e.g., ioxaglate), but also non-ionic monomers (e.g., ioversol, iohexol, iopamidol, iomeprol, iopromide). These CM are known as low-osmolar CM (LOCM), exhibiting osmolality lower than that of first-generation media, but still high compared with plasma (approximately 400-800 mOsm/L). Third-generation CM (e.g., iodixanol, iotrol), termed iso-osmolar CM (IOCM), are non-ionic dimers containing two benzene rings, with osmotic pressure approximately equal to plasma (290 mOsm/L) [1,10]. Some studies have reported slight differences in the safety of different types of CM; compared with ionic CM, non-ionic CM are associated with lower adverse reaction rates and less severe side effects [11-13]. At present, the most widely used and safe CM for cerebral angiographies are ioversol, iohexol and iodixanol.
Adverse reactions to iodinated CM
Although CM is regarded as relatively safe and effective drugs, severe adverse reactions may occur. They may affect either specific organs (such as the kidneys, heart, brain, glands) or a whole system, including inducing changes in blood components (platelets, leukocytes, erythrocytes) or endothelial cell function [10,14,15]. Adverse reactions to CM are generally divided into idiosyncratic reactions (i.e., anaphylactoid reactions) and dose-related effects on specific organ systems [3,16]. Specific manifestations of idiosyncratic reactions include urticaria, angioedema, laryngeal edema, shock, and bronchial spasm, and the occurrence of these symptoms does not generally have a relationship with the amount of CM [17]. In contrast, dose-related reactions largely depend on the amount of CM, and the toxic effects of the nervous system appear to be particularly strongly related to the dose [3]. The detrimental effects of CM fall into three major categories, depending on the time of appearance after administration of the contrast agent: acute (< 60 min), subacute (> 1 h to 7 d) and chronic (> 7 d). In addition, acute adverse reactions are classified as renal and non-renal, on the basis of whether kidney damage occurs; the latter typically involve nausea, vomiting, urticaria, shock, cardiac arrest and convulsions [8].
The pathogenesis of CIE
In 1970, Fischer-Williams et al. [4] first reported transient cortical blindness after coronary angiography. In later studies, Studdard [18], Haley [19], Utz [20] and Antonellis [21] reported similar cases following the administration of CM to the coronary artery, renal artery, and cerebral vascular system, and in other interventional procedures. Therefore, understanding the mechanisms underlying CIE is of increasing research importance. CIE is an acute, reversible neurological deficit that may present with psychiatric symptoms, transient cortical blindness, transient global amnesia, epilepsy and focal neurological deficits, which can be caused by various vascular interventional procedures involving the administration of iodinated CM [22,23]. Hypertension, diabetes and renal damage are considered possible risk factors [6,24]. In addition, subarachnoid hemorrhage, atherosclerosis and arteriovenous malformation, history of cardiovascular disease, fluoroscopic time longer than 10 minutes, and advanced patient age may have an increased risk of neurological complications [25-27]. It has been reported that the incidence of CIE ranges between 0.3% and 1.0%, and it can rise to 4% when hyperosmolar iodinated CM are used. Typical adverse reactions typically occur shortly after CM injection, and most can recover completely within 24- 48 h [5,6], while permanent neurological deficits or death occur in a small number of cases [24]. The diagnosis can be confirmed by the spontaneous and rapid resolution of the neurological symptoms and by CT findings [24]. Importantly, CIE is difficult to distinguish from other cerebrovascular complications, such as subarachnoid haemorrhage or cerebral ischaemia. But the difference is that CIE patients exhibiting serious neuroimaging changes often still have a relatively good prognosis [21,28,29].
The pathogenesis of CIE has not been fully elucidated, and a range of potential factors may be involved. First, CM may have direct chemical toxicity, hyperosmolality and neurotoxicity [30]. Second, the hyperosmolality of the media can induce hemodynamic changes, causing microvascular blood stasis and erythrocyte agglutination, resulting in slow blood flow and arterial occlusion [19]. Moreover, for patients with cerebrovascular disease, hemodynamic changes induced by rapid injection of CM can cause or aggravate cerebral vasospasm, leading to neurological deficits [18,31]. Third, a high concentration of CM can increase BBB permeability, and CM entering the brain can cause changes in osmotic pressure, leading to cerebral edema. Meanwhile the ionic properties of CM may also lead to changes in neural activity [30]. Several studies have reported that the pathogenesis of CIE may be related to the immune response of the body, indicating that iodinated CM have cytotoxic effects on immune cells [32]. In addition, the posterior circulation of the brain is more susceptible to damage by its sympathetic enervation, having greater sensitivity to blood pressure changes. In addition, the self-regulation ability of blood vessels in this area is poor, so general CM could easily permeate the parieto-occipital cortex, resulting in cortical blindness [5,33]. Importantly, Roza et al. [34] found that encephalopathy was more likely to occur in patients with acute renal injury. This suggests that renal and brain damage may be associated with oxidative stress and mitochondrial dysfunction caused by CM. Recent studies have reported that autophagy is involved in the pathogenesis of contrast-induced nephropathy by regulating apoptosis and inflammation [35]. Overall, although the precise mechanisms of CIE remain unclear, BBB permeability changes are generally considered to play an important role in CIE pathogenesis.
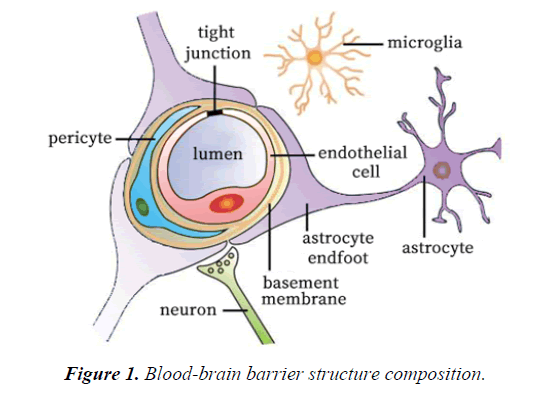
Structure and function of BBB
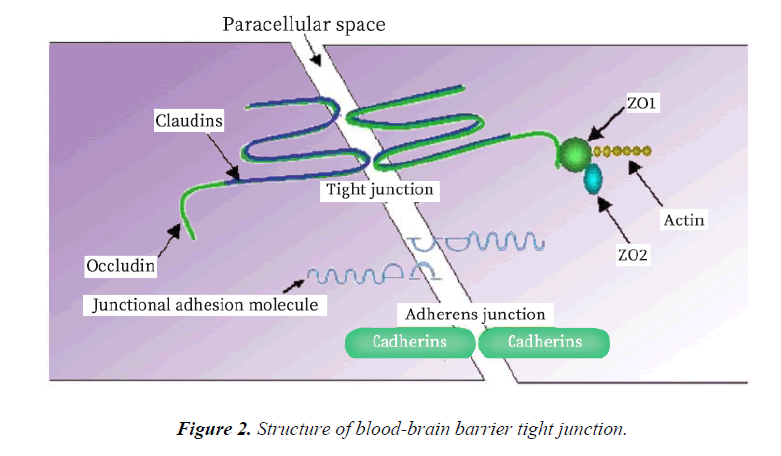
The BBB acts as an effective barrier between the central nervous system and the blood, and exists in all organisms with highly developed central nervous systems (CNS) [36]. The blood brain barrier controls the ion and water balance in the central nervous system and the level of neurotransmitters and hormones by maintaining the precise control of substance exchange in blood and brain, so as to maintain the homeostasis of brain microenvironment [37]. This physiological barrier maintains the steady state of the central nervous system in three ways: 1. the restriction of polar molecules passively diffusing from the blood to the brain; 2. regulating the transport of nutrients in the brain, while excreting toxic metabolites; and 3. regulating the migration of circulating immune cells [38-41]. The BBB is composed of brain microvascular endothelial cell, astrocyte endfoot, pericyte, tight junction (TJ) between brain microvascular endothelial cell, and the basement membrane (Figure 1 and Table 1). The intimate contact between neurons, cells, extracellular matrix components and blood vessels form a dynamic functional unit, known as the neurovascular unit, is essential for both health and function of the CNS [42,43]. Among these structures, TJ plays a particularly important role. TJ is constituted by a combination of transmembrane proteins, cytoplasmic scaffolding proteins and cytoskeleton proteins (Figure 2). The transmembrane proteins include occludin, claudins and junctional adhesion molecules, these proteins can play a different role in controlling the permeability of the intercellular space and maintaining the polarity of the endothelial cells [44]. Cytoplasmic scaffolding proteins (ZO family, cingulin, 7H6) are involved in TJ localization and signal transduction [45-47], and are directly bound to the intracellular carboxyl groups of transmembrane proteins and cytoskeletal proteins at the same time. The developmental and functional abnormalities of BBB can destroy the homeostasis of the microenvironment of the brain, leading to the death of the nerve cells and the dysfunction of the nervous system [48]. Ischemia, hypoxia, tumor, infection and other factors can cause structural and functional alterations of TJ, resulting in BBB permeability changes, brain edema, neuroinflammatory responses and neurological deficits (Table 2) [48-55].
| Structure composition | Function |
|---|---|
| Brain microvascular endothelial cell | Form tight junction to maintain structural stability of BBB. |
| Astrocyte | Astrocytes's endfeet closely apposed to the outer surface of the endothelial cell and pericyte. |
| Pericyte | Wrap around the endothelial cells to provide structural support and vasodynamic capacity to the microvasculature. |
| Tight junction | Located on the apical region of endothelial cells and form an intricate complex as a series of multiple barriers. |
| cytoplasmic scaffolding proteins | ZO family, cingulin, 7H6 |
| transmembrane proteins | occludin, claudins and junctional adhesion molecules |
| cytoskeleton proteins | Connect membrane proteins with actin, which is the primary cytoskeleton protein for the maintenance of structural and functional integrity of the endothelium. |
| Adherens junction | Composed of transmembrane glycoproteins linked to the cytoskeleton by cytoplasmatic proteins, giving place to the adhesion belt. |
| Basement membrane | The extracellular matrix of structural proteins secreted by astrocytes, pericytes and endothelial cells. |
Table 1. The main structure of blood-brain barrier and its function.
| Disease | Manifestation |
|---|---|
| Stroke | Focal cerebral ischemia damages elements of the BBB and induces inflammatory processes that alter the relationships of ECs, ECM, and astroglial cells. This results in profound changes in the microvascular permeability barrier. |
| Alzheimer disease | BBB destruction and Aβ scavenging ability decrease. |
| Parkinson's disease | Increased BBB permeability and decreased transport activity in BBB, such as the expression of P-gp transporter decreased significantly . |
| Multiple sclerosis | Reactive T cells recognize and destroy myelin sheath and the underlying axons, BBB function becomes compromised. |
| Amyotrophic lateral sclerosis | The breakdown of BBB can trigger oxidative stress and an autoimmune response, leading to demyelination, neurotransmission dysfunction and neuron death. |
| Brain tumor | The BBB is poorly developed in brain tumor leading to increased vascular permeability. And cerebral edema is an important consequence of brain tumor. |
Table 2. Central nervous systems diseases associated with blood-brain barrier dysfunction.
Role of BBB damage in the pathogenesis of CIE
As early as the 1940s, Broman et al. [56] observed the effects of CM on BBB permeability in rabbits, cats and guinea pigs using Trypan blue staining after administering the CM Diodrast, in an investigation of the mechanisms underlying CM neurotoxicity. The results revealed that cerebral vascular permeability was vulnerable to disturbance after injection of a high concentration of Diodrast to the carotid artery, for a certain period of time. Bassett et al. [57] later followed Borman’s method to observe the effects of different concentrations of CM on cerebrospinal fluid pressure and protein changes in animals (representing BBB destruction), reporting that the concentration and exposure time of CM were closely related to the severity of the effects. Almost twenty years later, Rapoport [58] observed the effects of different types of CM on the rabbit BBB, reporting that the neurotoxicity of CM was related to their chemical and physical properties, including permeability, fat solubility, and blood viscosity. With the advent of third-generation CM, the choice of CM has increased, but how to choose the proper one becomes a difficult problem. A large-scale clinical study involving 337,647 participants confirmed that, compared with ionic CM, non-ionic CM were associated with a lower incidence of adverse reactions, and milder side effects [11]. Wible and his colleagues [59] compared the neurotoxicity of four kinds of nonionic CM (ioversol, iohexol, iopamidol, iopromide) by injecting different types and doses of CM into the cisternal magna of ether-anesthetized rats, demonstrating that the neurotoxicity of the media was negatively correlated with its hydrophilicity. In contrast, Luzzani et al. [60] found that differences in hydrophilicity between CM did not explain observed differences in neurotoxicity in animal experiments, suggesting that the chemical structure of CM may play a role. Iso-osmolar CM are typically considered the safest CM type, compared with hypertonic and hypotonic media. However, in 2011, Chisci [28] first reported a CIE patient exhibiting aphasia, coma and hemiplegia after administration of iodixanol. Therefore, to fully understand the pathogenesis of CIE, multiple factors must be considered.
Over the past twenty years, many scholars have described cases of transient cortical blindness or amnesia after angiography, and speculated that possible mechanism are ischemia to bilateral limbic structures (from vasospasm, atherosclerotic plaque, catheter-induced embolus) and direct neurotoxic effects of the CM. Unsurprisingly, CM are considered to have neurotoxic effects by temporarily disrupting the BBB with subsequent parenchymal penetration [26,61-66]. In fact, a number of studies have investigated the mechanisms by which CM affect BBB permeability and cause encephalopathy. Some authors have advocated that intravascular pressure arising from rapid injection of CM, may cause increasing vascular wall tension and separation of tight junctions leading to a breakdown of the BBB [3,67,68]. However, several authors have favored that alterations on endothelial cells of brain vessels caused by CM as the key factor leading to BBB damage. Gonsette et al. [69] explored the safety of first and second generation CM in animal experiments, suggesting at least two mechanisms by which CM may damage the BBB: 1. the chemical toxicity of CM may promote an increase in endocytosis and exocytosis of microvascular endothelial cells in the brain, as well as weakening the enzymatic reaction; 2. hyperosmolality may cause endothelial cells to shrink and destroy the integrity of TJ. In 2008 Franke et al. [70] added CM to the culture medium of human umbilical venous endothelial cells and used for short-term incubation studies of these cells, finally found CM can cause relevant morphological changes in endothelial cells. Aspelin et al. [71] reported that CM may induce endothelial cell injury by inhibiting endothelial cell growth and promoting apoptosis. Chang et al. [72] found that CM induces inflammatory responses in endothelial cells in their researches. Moreover, CM may also promote vasoconstriction by inhibiting endothelial nitric oxide synthase activity and expression. This may impede nitric oxide synthesis, further reducing nitric oxide by generating reactive oxygen species (ROS) in response to induced endothelial dysfunction and inflammatory responses, thereby reducing prostacyclin and endothelium-derived hyperpolarizing factor release while increasing endothelin-1 and adenosine release [10,68]. Rauch et al. compared the effects of CM in rabbit, dog, and pig renal arteries with human tissue. CM-induced vasoconstriction was demonstrated in human, rabbit and dog, whereas vasodilatation was observed in pig [73]. In general, the presence of CM in blood circulation has been found to change the morphology and function of endothelial cells, which is an important structure of blood-brain barrier. The damaged endothelial cells make TJ open, allowing CM to enter into the central nervous system and change the osmotic pressure of brain tissue, causing cerebral edema. Meanwhile, damaged endothelial cells may aggravate the conditions described above by stimulating the release of a variety of vasoactive substances. In addition, the effects of CM on neural electrical activity may also be involved in the processes underlying neural damage.
It has been hypothesized that oxidative stress increases after application of CM, and excessive production of free radicals can cause damage to endothelial cells. Fiaccadori [74] found that the concentration of 3-nitrotyrosine in plasma and urine of patients with CM was significantly increased, which indirectly confirmed the important role of oxygen free radicals in the pathogenesis. Some studies in vitro indicate that NADPH oxidase plays a key role in upregulation of ROS in a cellular model added CM [75]. Several studies have confirmed that antioxidants such as statins and vitamins can alleviate the cytotoxicity induced by CM via reducing the expression of NADPH oxidase subunit and production of oxygen free radicals [76,77]. Given these considerations, we suggest that oxidative stress is closely related to the destruction of BBB in the pathogenesis of CIE, and is a potential target for therapeutic intervention.
Conclusion
In conclusion, CIE is a rare complication of vascular intervention, and its pathogenesis has not yet been fully elucidated. While BBB damage appears to play a major role in the condition, it remains to be clarified whether oxidative stress, mitochondrial dysfunction and autophagy are also important factors.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Acknowledgement
We would like to express our gratitude for our department colleagues’ assistance.
Disclosure Statement
None of the authors has any conflict of interest related to this manuscript.
References
- Bottinor W, Polkampally P, Jovin I. Adverse reactions to iodinated contrast media. Int J Angiol. 2013;22:149-54.
- Beckett KR, Moriarity AK, Langer JM. Safe Use of Contrast Media: What the Radiologist Needs to Know. Radiographics. 2015;35:1738-50.
- Junck L, Marshall WH. Neurotoxicity of radiological contrast agents. Ann Neurol. 1983;13:469-84.
- Fischer-Williams M, Gottschalk PG, Browell JN. Transient cortical blindness. An unusual complication of coronary angiography. Neurology. 1970;20:353-5.
- Potsi S, Chourmouzi D, Moumtzouoglou A, et al. Transient contrast encephalopathy after carotid angiography mimicking diffuse subarachnoid haemorrhage. Neurol Sci. 2012;33:445-8.
- Spina R, Simon N, Markus R, et al. Recurrent contrast-induced encephalopathy following coronary angiography. Intern Med J. 2017;47:221-4.
- Morcos SK, Thomsen HS, Exley CM. Contrast media: interactions with other drugs and clinical tests. Eur Radiol. 2005;15:1463-8.
- Thomsen HS. Contrast media safety-an update. Eur J Radiol. 2011;80:77-82.
- Reiner JS. Contrast media and clotting: what is the evidence? Catheter Cardiovasc Interv. 2010;75:S35-8.
- Scoditti E, Massaro M, Montinari MR. Endothelial safety of radiological contrast media: why being concerned. Vascul Pharmacol. 2013;58:48-53.
- Katayama H, Yamaguchi K, Kozuka T, et al. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990;175:621-8.
- Rose TA, Choi JW. Intravenous Imaging Contrast Media Complications: The Basics That Every Clinician Needs to Know. Am J Med. 2015;128:943-9.
- Kim KH, Park JY, Park HS, et al. Which iodinated contrast media is the least cytotoxic to human disc cells? Spine J. 2015;15:1021-7.
- Albanese JR, Venditto JA, Patel GC, et al. Effects of ionic and nonionic contrast media on in vitro and in vivo platelet activation. Am J Cardiol. 1995;76:1059-63.
- Ramponi S, Grotti A, Morisetti A, et al. Effects of iodinated contrast media on endothelium: An in vitro study. Toxicol In Vitro. 2007;21:191-6.
- Davis PL. Anaphylactoid reactions to the nonvascular administration of water-soluble iodinated contrast media. AJR Am J Roentgenol. 2015;204:1140-5.
- Lieberman P, Siegle RL, Taylor WW. Anaphylactoid reactions to iodinated contrast material. J Allergy Clin Immunol. 1978;62:174-80.
- Studdard WE, Davis DO, Young SW. Cortical blindness after cerebral angiography. Case report. J Neurosurg. 1981;54:240-4.
- Haley EC Jr. Encephalopathy following arteriography: a possible toxic effect of contrast agents. Ann Neurol. 1984;15:100-2.
- Utz R, Ekholm SE, Isaac L, et al. Local blood-brain barrier penetration following systemic contrast medium administration. A case report and an experimental study. Acta Radiol. 1988;29:237-42.
- Antonellis J, Kostopoulos K, Rambaouni A, et al. Cortical blindness following coronary arteriography: a rare but self-cured complication. Two case reports. Angiology. 1996;47:803-6.
- Frontera JA, Pile-Spellman J, Mohr JP. Contrast-induced neurotoxicity and selective cortical injury. Cerebrovasc Dis. 2007;24:148-51.
- Liao MT, Lin TT, Lin LY, et al. Contrast-Induced Encephalopathy after Percutaneous Coronary Intervention. Acta Cardiol Sin. 2013;29:277-80.
- Kocabay G, Karabay CY, Kalayci A, et al. Contrast-induced neurotoxicity after coronary angiography. Herz. 2014;39:522-7.
- Kaufmann TJ, Huston J, Mandrekar JN, et al. Complications of diagnostic cerebral angiography: evaluation of 19,826 consecutive patients. Radiology. 2007;243:812-9.
- Tiu C, Terecoasa EO, Grecu N, et al. Transient Global Amnesia After Cerebral Angiography With Iomeprol: A Case Report. Medicine (Baltimore). 2016;95:e3590.
- Willinsky RA, Taylor SM, TerBrugge K, et al. Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology. 2003;227:522-8.
- Chisci E, Setacci F, de Donato G, et al. A Case of Contrast-Induced Encephalopathy Using Iodixanol. Journal of Endovascular Therapy. 2011;18:540-4.
- Leong S, Fanning NF. Persistent neurological deficit from iodinated contrast encephalopathy following intracranial aneurysm coiling. A case report and review of the literature. Interv Neuroradiol. 2012;18:33-41.
- Yu J, Dangas G. New Insights Into the Risk Factors of Contrast-Induced Encephalopathy. Journal of Endovascular Therapy. 2011;18:545-6.
- Prendes JL. Transient cortical blindness following vertebral angiography. Headache. 1978;18:222-4.
- O'Donnell DH, Moloney MA, Bouchier-Hayes DJ, et al. Iodinated contrast media alter immune responses in pro-inflammatory states. Acta Radiol. 2010;51:635-40.
- Guimaraens L, Vivas E, Fonnegra A, et al. Transient encephalopathy from angiographic contrast: a rare complication in neurointerventional procedures. Cardiovasc Intervent Radiol. 2010;33:383-8.
- Roza CA, Scaini G, Jeremias IC, et al. Evaluation of brain and kidney energy metabolism in an animal model of contrast-induced nephropathy. Metab Brain Dis. 2011;26:115-22.
- Ko GJ, Bae SY, Hong YA, et al. Radiocontrast-induced nephropathy is attenuated by autophagy through regulation of apoptosis and inflammation. Human & Experimental Toxicology. 2016;35:724-36.
- Abbott NJ. Dynamics of CNS Barriers: Evolution, Differentiation, and Modulation. Cellular and Molecular Neurobiology. 2005;25:5-23.
- Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584-96.
- Luissint AC, Artus C, Glacial F, et al. Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids Barriers CNS. 2012;9:23.
- Abbott NJ, Patabendige AA, Dolman DE, et al. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13-25.
- Begley DJ, Brightman MW. Structural and functional aspects of the blood-brain barrier. Prog Drug Res. 2003;61:39-78.
- Wolburg H, Noell S, Mack A, et al. Brain endothelial cells and the glio-vascular complex. Cell Tissue Res. 2009;335:75-96.
- Muoio V, Persson PB, Sendeski MM. The neurovascular unit - concept review. Acta Physiol (Oxf). 2014;210:790-8.
- Serlin Y, Shelef I, Knyazer B, et al. Anatomy and physiology of the blood-brain barrier. Semin Cell Dev Biol. 2015;38:2-6.
- Furuse M. Molecular basis of the core structure of tight junctions. Cold Spring Harb Perspect Biol. 2010;2:a002907.
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1-13.
- Cardoso FL, Brites D, Brito MA. Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain Res Rev. 2010;64:328-363.
- Keaney J, Campbell M. The dynamic blood-brain barrier. FEBS J. 2015;282:4067-79.
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178-201.
- Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7:a020412.
- Groothuis DR, Vriesendorp FJ, Kupfer B, et al. Quantitative measurements of capillary transport in human brain tumors by computed tomography. Ann Neurol. 1991;30:581-8.
- Hirano S, Asanuma K, Ma Y, et al. Dissociation of metabolic and neurovascular responses to levodopa in the treatment of Parkinson's disease. J Neurosci. 2008;28:4201-9.
- Larochelle C, Alvarez JI, Prat A. How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett. 2011;585:3770-80.
- Saadoun S, Papadopoulos MC, Davies DC, et al. Aquaporin-4 expression is increased in oedematous human brain tumours. Journal of Neurology Neurosurgery and Psychiatry. 2002;72:262-5.
- Tanzi RE, Moir RD, Wagner SL. Clearance of Alzheimer's Abeta peptide: the many roads to perdition. Neuron. 2004;43:605-8.
- Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323-37.
- Broman T, Olsson O. The tolerance of cerebral blood-vessels to a contrast medium of the Diorast group: An experimental study of the effect on the blood-brain-barrier. Acta Radiol. 1948;30:326-42.
- Bassett RC, Rogers JS, Cherry GR, et al. The effect of contrast media on the blood-brain-barrier. J Neurosurg. 1953;10:38-47.
- Rapoport SI, Thompson HK, Bidinger JM. Equi-osmolal opening of the blood-brain barrier in the rabbit by different contrast media. Acta Radiol Diagn(Stockh). 1974;15:21-32.
- Wible JH Jr., Barco SJ, Scherrer DE, et al. Neurotoxicity of non-ionic X-ray contrast media after intracisternal administration in rats. Eur J Radiol. 1995;19:206-11.
- Luzzani F, Morisetti A, Bussi S, et al. Neurotolerability of nonionic X-ray contrast media. The role of chemotoxicity. Invest Radiol. 1996;31:338-44.
- Chen CY, Chen CJ, Tseng YC. A Case Report of Transient Cortical Blindness After Angiography. Neurologist. 2017;22:82-4.
- Giang DW, Kido DK. Transient global amnesia associated with cerebral angiography performed with use of iopamidol. Radiology. 1989;172:195-6.
- Juni J, Morera J, Lainez JM, et al. Transient global amnesia after cerebral angiography with iohexol. Neuroradiology. 1992;34:141-3.
- Lo LW, Chan HF, Ma KF, et al. Transient cortical blindness following vertebral angiography: a case report. Neurointervention. 2015;10:39-42.
- Niimi Y, Kupersmith MJ, Ahmad S, et al. Cortical blindness, transient and otherwise, associated with detachable coil embolization of intracranial aneurysms. AJNR Am J Neuroradiol. 2008;29:603-7.
- Roccatagliata L, Taveira-Lopes L, Rossignol MD, et al. Cortical Blindness and Retrograde Amnesia Following Cerebral Angiography Studied by Early Diffusion Weighted MR imaging. A Case Report. Neuroradiol J. 2009;22:600-4.
- Spina R, Simon N, Markus R, et al. Contrast-induced encephalopathy following cardiac catheterization. Catheter Cardiovasc Interv. 2017;90:257-68.
- Torvik A, Walday P. Neurotoxicity of water-soluble contrast media. Acta Radiol Suppl. 1995;399:221-9.
- Gonsette RE, Liesenborgh L. New Contrast-Media in Cerebral-Angiography - Animal-Experiments and Preliminary Clinical-Studies. Investigative Radiology. 1980;15:S270-4.
- Franke RP, Fuhrmann R, Hiebl B, et al. Influence of various radiographic contrast media on the buckling of endothelial cells. Microvasc Res. 2008;76:110-3.
- Aspelin P, Stacul F, Thomsen HS, et al. Members of the Contrast Media Safety Committee of the European Society of Urogenital R. Effects of iodinated contrast media on blood and endothelium. Eur Radiol. 2006;16:1041-9.
- Chang CF, Liu XM, Peyton KJ, et al. Heme oxygenase-1 counteracts contrast media-induced endothelial cell dysfunction. Biochem Pharmacol. 2014;87:303-11.
- Rauch D, Drescher P, Pereira FJ, et al. Comparison of iodinated contrast media-induced renal vasoconstriction in human, rabbit, dog, and pig arteries. Invest Radiol. 1997;32:315-9.
- Fiaccadori E, Maggiore U, Rotelli C, et al. Plasma and urinary free 3-nitrotyrosine following cardiac angiography procedures with non-ionic radiocontrast media. Nephrol Dial Transplant. 2004;19:865-9.
- Netti GS, Prattichizzo C, Montemurno E, et al. Exposure to low- vs iso-osmolar contrast agents reduces NADPH-dependent reactive oxygen species generation in a cellular model of renal injury. Free Radic Biol Med. 2014;68:35-42.
- Liu GL, Lei R, Duan SB, et al. Atorvastatin alleviates iodinated contrast media-induced cytotoxicity in human proximal renal tubular epithelial cells. Exp Ther Med. 2017;14:3309-13.
- Naziroglu M, Yoldas N, Uzgur EN, et al. Role of contrast media on oxidative stress, Ca(2+) signaling and apoptosis in kidney. J Membr Biol. 2013;246:91-100.