Research Article - Biomedical Research (2017) Volume 28, Issue 17
The repair effect of Huoxue Tongdu decoction on motor function in rat models of acute spinal cord injury via NFs/Trk pathway
Qingbin Lin and Li Zhang*
Fujian University of Traditional Chinese Medicine, Fuzhou, PR China
Accepted on August 03, 2017
Abstract
Objective: To study the effect of Huoxue Tongdu decoction on motor function in rat models of acute Spinal Cord Injury (SCI) via NFs/Trk pathway.
Method: Totally 108 SD rats were randomly divided into Sham operation group (36 cases), Model group (36 cases) and Huoxue Tongdu decoction treatment group (36 cases). BBB score was evaluated for the hind limbs before surgery and at 1 d, 3 d, 5 d and 7 d after surgery, randomly gave 6 rats termination in each group. The spinal cord was harvested and subjected to HE staining and Nissl staining for observing the morphological changes of motor neurons in the anterior gray horn. The expression levels of BDNF and NT-3 were detected by immunofluorescence assay, and the cells positive for the expression of these two proteins were counted. Western blot was performed to detect the expression of Trk B and Trk C receptors.
Result: Compare with the Model group, BBB score at 1 d, 3 d, 5 d and 7 d in Huoxue Tongdu decoction group was much better (P<0.05). The differences of percentage of Nissl staining positive areas between Model group and Huoxue Tongdu decoction group showed statistical significance (P<0.05). The expression levels of BDNF, NT3 and proteins related to NFs/Trk pathway were significantly up-regulated in Huoxue Tongdu decoction treatment group compared with that in Sham operation group and Model group (P<0.05). The expression levels of Trk B and Trk C receptors in Huoxue Tongdu decoction treatment group, were significantly up-regulated as compared with the Model group (P<0.05). Motor neurons of the anterior gray horn showed good morphology in Huoxue Tongdu decoction.
Conclusion: Huoxue Tongdu decoction promoted the repair of motor function in rats with SCI via the NFs/Trk pathway, which may be beneficial for the survival and axonal repair of neurons.
Keywords
Spinal cord injury (SCI), Huoxue Tongdu decoction, Neurons, NFs/Trk pathway.
Introduction
Spinal Cord Injury (SCI) consists of the primary and secondary type [1]. So far, no treatment can completely cure SCI, and medical provision for patients with SCI was usually met with frustration, bringing great burden to the patients as well as the society [2,3]. In America, up to 1.275 million patients live with SCI and nearly 20,000 new cases are increasing yearly [4]. SCI is a complex pathophysiology, depending on the site and severity of lesion along the spinal cord, the symptoms can vary widely, which includes edema and ischemia of spinal cord, ion imbalance and electrolyte disturbance, toxicity of excitatory amino acids, inflammation, cell necrosis and apoptosis. SCI will lead to loss of muscle function, sensation, or autonomic function served by the spinal cord below the level of the lesion [5].
Chinese medicinal herbs used for medication were more than one thousand years, Huoxue Tongdu decoction is an experiential prescription invented by Anzhen, a Chinese expert in orthopaedics. This prescription is now widely used to treat Spinal Cord Injury (SCI) with attractive treatment effects [6]. The main ingredients of the prescription are Astragalus mongholicus, Angelica sinensis and Salvia miltiorrhiza. These Chinese medicinal herbs can promote blood circulation and remove blood stasis [7]. So this decoction should be applied to bone fractures and nerve injury. Neurotrophic Factors (NF) are polypeptides which can maintain cell survival, promote cell growth and differentiation and have other cell functions [8]. NFs play an important role in spinal cord injury recovery, neuron survival and regeneration, and synaptic reconstruction following SCI, which can be depended to realize the motor function of the limbs [9,10]. This study aimed to clarify the working mechanism of Huoxue Tongdu decoction for SCI by focusing on its effect on NFs.
Materials and Method
Method
(1) Grouping: Total of 108 SD clean-grade rats, including male and female, were randomly and equally divided into 3 groups, Sham operation group (n=36), Model group (n=36) and Huoxue Tongdu decoction treatment group (n=36), all rats were anesthetized by intra-peritoneal injection with 10% chloralic hydras. Each group was subdivided into 4 subgroups by the time of survival (1d, 3d, 5d and 7d); (2) Rats in Sham operation group only received laminectomy at T10 without injuring the spinal cord. Intragastric infusion of normal saline was performed after the rats woke up from anesthesia. BBB score was evaluated before surgery and at 1 d, 3 d, 5 d and 7 d after surgery, respectively. The rats were sacrificed and the spinal cords were harvested at 1 d, 3 d, 5 d and 7 d after surgery; (3) Rats in the Model group received laminectomy at T10 and SCI which was induced by using modified Allen’s method. For Huoxue Tongdu decoction treatment group, laminectomy was performed at T10, and followed by modified Allen’s method to induce SCI. After the rats woke up from anesthesia, intragastric infusion of Huoxue Tongdu decoction was performed. BBB score was evaluated at 1 d, 3 d, 5 d and 7 d after surgery respectively. Then the rats were sacrificed and the spinal cord was harvested; (4) HE staining and Nissl staining were performed to visualize the morphology changes of neurons. Cells which were positive for BDNF and NT-3 were detected and localized by using immunofluorescence assay. The positive cell rate was determined by semi-quantitative analysis. Western blot detection was used to detect the expression of Trk B and C receptors; (5) Nissl bodies can be stained purple blue by Nissl staining, especially in the brain and spinal cord neurons. The presence of large and massive Nissl bodies usually indicates strong protein synthesis in the neurons. In contrast, Nissl bodies will decrease in quantity or even disappear in the case of neuronal damage. According to this criterion, the Nissl staining at 1 d, 3 d, 5 d and 7 d after surgery was observed under the inverted microscope (200x).
Materials: Taq Master Mix (SinoBio, USA), agarose (Biowest), sterile double-distilled water, p-Trk receptor B (1:1,000; Cell Signaling, USA), anti-Trk B antibody (1:5,000; Invitrogen, USA), 0.9% sterile normal saline (Otsuka Pharmaceutical Co., Ltd. , Japan).
Equipments: PCR instrument (Bio-Rad, USA), gel imager (Bio-Rad, USA), electrophoresis apparatus (Beijing Liuyi Instrument Factory), centrifuge (Eppendorf, Germany), micropipettor (Eppendorf, Germany), ice machine (Hair, China), Western Blot system (Bio-Rad, USA), -80°C fridge (Thermo, USA), 10 ml syringe, 5 ml syringe (Hanaco Medical, Tianjin), special surgical instruments for laboratory animals (Beijing Medical Instrument Factory), NanoDrop2000 spectrophotometer (Thermo, USA), EP tube (Eppendorff, Germany), water bath kettle (Beijing Medical Instrument Factory), microtome (Leica, Germany).
Judgment of the result
BBB rating was performed to evaluate the motor function after SCI. BBB score ranged from 1 to 21. The higher the score was, the better the motor function in the hind limbs was [8].
Ingredients of Huoxue Tongdu decoction
Astragalus mongholicus 18 g, Angelica sinensis 9 g, Salvia miltiorrhiza 18 g, Eucommia ulmoides 9 g, myrrh 9 g, earthworm 9 g, Caesalpinia sappan 9 g, herba lycopi 9 g, Rhizoma cibotii 12 g, antler 18 g, twice a day.
Statistical method
All the data was represented by (Mean value and Standard deviation, ͞x ± ta). Statistical analysis was performed by using the SPSS22.0 statistical software. The Kruskal-Wallis-H test was used to compare the data of multiple groups, non-parametric numerical data. The Wilcoxon test was used for statistical analysis of changes during follow-up within the groups (Sham operation group, Model group, Huoxue Tongdu decoction group). The two group test data by using LSD. Non-normal distribution or homogeneity of variance used nonparametric test or one-way ANOVA of Dunnett’s T3 method. When P<0.05, it means that the difference was considered to be statistically significant.
Result
Comparison of BBB score among three groups
Before treatment, the three groups showed no significant difference in BBB score. The result of Sham operation group was not significantly different before and after treatment. For the Model group and Huoxue Tongdu decoction treatment group, BBB score decreased significantly as compared with the Sham operation group (P<0.05). BBB score at 1 d, 3 d, 5 d and 7 d in Huoxue Tongdu decoction group was much higher than that of the Model group (P<0.05). Rats in Huoxue Tongdu decoction group recovered faster after surgery, as compared with the Model group (P<0.05) (Table 1).
| Time | Sham operation (n=36) |
Model (n=36) |
Huoxue Tongdu decoction (n=36) |
F | P |
|---|---|---|---|---|---|
| Before treatment | 19.55 ± 1.02 | 19.78 ± 0.99 | 19.44 ± 1.07 | 1.03 | 0.36 |
| At 1 d after treatment | 19.33 ± 1.18 | 3.44 ± 1.26m | 5.08 ± 1.13mn | 1937.65 | 0 |
| At 3 d after treatment | 19.39 ± 1.22 | 4.18 ± 1.29m | 8.31 ± 0.85mn | 1724.13 | 0 |
| At 5 d after treatment | 19.42 ± 1.05 | 6.26 ± 1.42m | 12.69 ± 1.33mn | 956.84 | 0 |
| At 7 d after treatment | 19.47 ± 1.19 | 7.41 ± 1.21m | 15.85 ± 1.42mn | 844.68 | 0 |
| Note: Compare with the Sham operation group, mP<0.05; Compare with the Model group, nP<0.05. | |||||
Table 1. Comparison of BBB scores among three groups of mice ((͞x s), %).
Comparison of the percentage of Nissl staining positive areas
Neuronal damage in Huoxue Tongdu decoction treatment group was greatly relieved as compared with the Model group (P<0.05) (Table 2).
| Time | Model group V (n=36) | Huoxue Tongdu decoction group (n=36) | t | P |
|---|---|---|---|---|
| Before treatment | 48.55 ± 2.44 | 47.36 ± 2.89 | 1.888 | 0.063 |
| At 1 d after treatment | 3.57 ± 0.94 | 6.28 ± 1.14 | 11.005 | 0 |
| At 3 d after treatment | 8.74 ± 1.83 | 13.17 ± 1.62 | 10.875 | 0 |
| At 5 d after treatment | 9.02 ± 1.49 | 31.74 ± 1.55 | 63.404 | 0 |
| At 7 d after treatment | 9.57 ± 1.36 | 33.86 ± 1.46 | 73.042 | 0 |
Table 2. Comparison of the percentage of Nissl staining positive areas between Model group and Huoxue Tongdu decoction group ((͞x s), %).
Comparison of detection and localization of cells positive for BDNF and NT-3
Cells which are positive for BDNF and NT-3 in the Model group and Huoxue Tongdu decoction treatment group were detected and localized by the immunofluorescence assay.
The number of BDNF-positive cells in Huoxue Tongdu decoction treatment group was significantly higher than that in the Model group (P<0.05). Both BDNF and NT-3 were localized to the neurons in the spinal cord (Table 3).
| Time | Model group (n=36) | Huoxue Tongdu decoction group (n=36) | t | P |
|---|---|---|---|---|
| Before treatment | ||||
| BDNF | 20.17 ± 2.35 | 21.06 ± 2.12 | 1.687 | 0.096 |
| NT-3 | 18.73 ± 4.76 | 17.49 ± 4.58 | 1.126 | 0.264 |
| At 1 d after treatment | ||||
| BDNF | 4.29 ± 0.83 | 7.39 ± 1.15 | 13.115 | 0 |
| NT-3 | 3.17 ± 0.62 | 5.29 ± 0.98 | 10.969 | 0 |
| At 3 d after treatment | ||||
| BDNF | 5.62 ± 1.95 | 10.77 ± 1.84 | 11.525 | 0 |
| NT-3 | 4.72 ± 1.19 | 7.33 ± 1.45 | 8.348 | 0 |
| At 5 d after treatment | ||||
| BDNF | 7.36 ± 2.14 | 20.31 ± 2.27 | 24.906 | 0 |
| NT-3 | 6.27 ± 1.64 | 10.28 ± 2.01 | 9.275 | 0 |
| At 7 d after treatment | ||||
| BDNF | 10.05 ± 1.74 | 22.17 ± 1.63 | 30.501 | 0 |
| NT-3 | 11.59 ± 2.64 | 21.91 ± 2.53 | 16.934 | 0 |
Table 3. Comparison of detection and localization of cells which are positive for BDNF and NT-3 between Model group and Huoxue Tongdu decoction group ((͞x s), %).
Expression of Trk B and Trk C receptors in the spinal cord
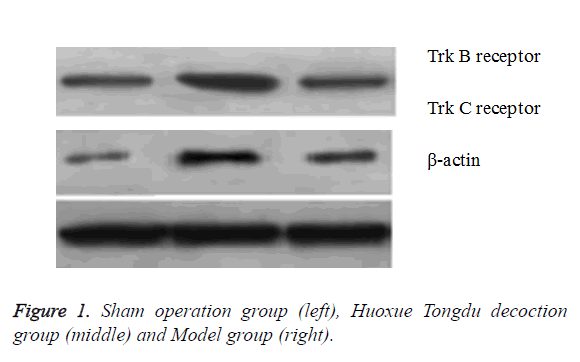
Expression levels of Trk B and Trk C receptors were detected by Western blot after surgery in order to study the effect of Huoxue Tongdu decoction. In Huoxue Tongdu decoction treatment group, the expression levels of Trk B and Trk C receptors were significantly up-regulated as compared with the Model group (P<0.05). This implied the important role of Huoxue Tongdu decoction in increasing the expression of Trk B and Trk C receptors (Table 4 and Figure 1).
| Groups | Trk B receptor | Trk C receptor | β-actin |
|---|---|---|---|
| Sham operation group (n=36) | 0.57 ± 0.13 | 0.41 ± 0.17 | 1 |
| Model group (n=36) | 0.42 ± 0.16 | 0.35 ± 0.19 | 1 |
| Huoxue Tongdu decoction group (n=36) | 0.94 ± 0.26mn | 0.88 ± 0.21mn | 1 |
| F | 70.267 | 83.384 | - |
| P | 0 | 0 | - |
| Note: Compare with the Sham operation group, mP<0.05; Compare with the Model group, nP<0.05. | |||
Table 4. Expression of Trk B and Trk C receptors in the spinal cord among three groups of mice ((͞x s), %).
Discussion
SCI is usually caused by external impact that acts on the spinal cord either directly or indirectly, leading to motor and sensory dysfunction, sphincter of oddi dysfunction, abnormal tension of muscle and other pathological changes [11,12]. The severity and symptoms of SCI depend on the position and features of the primary lesions. Traumatic SCI is mainly seen in adults, causing the adverse consequences of dyskinesia combined with and multiple lesions. SCI is difficult to treat and featured by high incidence of complications and disability rates. Therefore, the patients may suffer from physical and physiological trauma if not properly treated [13-15]. A series of cellular and molecular events may take place after SCI, including primary and secondary damage pathways. Much more severe pathological changes occur due to the magnification effect through secondary damage, which is usually associated with the activated inflammatory cascade [4-6]. Either primary or secondary SCI may lead to paraplegia or event death. Research shows that local or systemic inflammatory response will occur following SCI [6]. The expression levels of some genes are upregulated after SCI and mediate the NF-κB and AMPK signalling pathways. SCI is usually accompanied with massive release of nerve growth factors (NGFs), which are among the first discovered neurotrophins. NGFs have been intensively studied and regulate nerve growth, with neurotrophic effects and promoting effects on synaptic growth. NGFs can be found in central nervous system and peripheral neurons, promoting the development, differentiation and regeneration of neurons. The major types of neurotrophins include NGF, BDNF (Brain- Derived Neurotrophic Factor), basic Fibroblast Growth Factor (bFGF), Ciliary Neurotrophic Factor (CNTF) and NT-3 (Neurotrophin-3) [16-19].
Relevant studies have shown that Huoxue Tongdu decoction can be used to treat SCI via multiple pathways [7-9]. The main ingredients of Huoxue Tongdu decoction include Astragalus mongholicus, Angelica sinensis, Salvia miltiorrhiza, Eucommia ulmoides, myrrh, earthworm, Caesalpinia sappan, herba lycopi, Rhizoma cibotii and antler. This decoction has an obvious treatment effect on bone fractures and nerve damage through the following pathways: Firstly, Huoxue Tongdu decoction inhibits the apoptosis of spinal cord nerve cells by reducing the expression of c-Fos and c-Jun proteins in the spinal cord, thus relieving the spinal cord ischemia-reperfusion injury and promoting motor function recovery. secondly, the decoction which suppresses inflammatory responses by down-regulating the expression of TNF-α, IL-1β and IL-8 in the spinal cord, thus relieving SCI. Thirdly, the decoction which relieves inflammatory responses by suppressing the expression of NF-κB and VCAM-1, thus relieving the spinal cord ischemia-reperfusion injury and promoting the recovery after nerve damage.
In this work, SCI was induced by modified AllenSCI was i and BBB score was evaluated for the Model group, Sham operation group and Huoxue Tongdu decoction treatment group. BBB score of the Model group and the Huoxue Tongdu decoction treatment group decreased significantly as compared with the Sham operation group, indicating that the modelling was successful (P<0.05). At 1 d after surgery, the Model group and Huoxue Tongdu decoction treatment group did not show significant difference in BBB score; at 3 d, 5 d and 7 d, the BBB score of the Huoxue Tongdu decoction treatment group was significantly higher than that in the Model group (P<0.05). Thus Huoxue Tongdu decoction was effective in treating SCI and preventing subsequent damage of the normal tissues. The neurons were subjected to Nissl staining. It was found that neuronal damage occurred to varying extent in the Model group and Huoxue Tongdu decoction treatment group and the neural damage was significantly relieved in Huoxue Tongdu decoction treatment group as compared with the Model group (P<0.05). This indicated the protective effect of Huoxue Tongdu decoction for SCI on a pathological level. To clarify the working mechanism, the expression of BDNF and NT-3 was detected and localized by immunofluorescence assay. The number of cells which are positive for BDNF and NT-3 was significantly higher in Huoxue Tongdu decoction treatment group than that in the Model group (P<0.05). This result was consistent with previous findings. For example, Zhang et al. achieved satisfactory outcome by using Huoxue Tongdu decoction to treat spinal cord ischemia-reperfusion injury in rabbit models [9].
BDNF and NT-3 are mainly present in neurons of the spinal cord. As members of NGF family, BDNF and NT-3 are only expressed at a low level under normal conditions. But they are up-regulated under the action of various inflammatory factors following neuronal damage, which activates the neuronal protection mechanism via the upstream and downstream signalling pathways. While preserving the damaged neurons, the neuronal regeneration is promoted. Moreover, higher the expression of NGFs, the faster the recovery from nerve damage will be. Self-repair capacity of the nerve cells is only possible within a certain limit, and the up-regulation of NGFs will facilitate self-repair to the largest extent. The results of the present study accorded with our expectations.
Further experiment indicated that Huoxue Tongdu decoction exerted a neuroprotective effect by up-regulating Trk B and Trk C receptors. Trk receptor family consists of Receptor Tyrosine Kinases (RTKs) that regulate the intensity and plasticity of synapses in mammals. The activated Trk receptors influence the survival and differentiation of neurons via multiple signalling pathways. The common ligands of Trk receptors are neurotrophins, and the binding between the two groups is highly specific [2,20]. Each neurotrophin binds to a different Trk receptor with different affinity, which results in the regulation of cell survival and other functions. We believe that Huoxue Tongdu decoction achieved a neuroprotective effect by up-regulating Trk receptors in the spinal cord and promoting the binding between Trk receptors and neurotrophins.
To conclude, Huoxue Tongdu decoction promotes the recovery of motor function in rat models of SCI via the NFs/Trk pathway. The up-regulation of BDNF, NGF, NT-3 and the corresponding Trk receptors is beneficial for the survival, regeneration and axonal repair of neurons and for the recovery of motor function following SCI.
References
- Mo Y, Lv W, Yao HJ. Alteration of locomotor function and NT-3 and Trk C expression in the 14th day post-Spinal Cord Injury Rat and effect of different electro-acupuncture. Integr Med Res 2015; 4: 68.
- Song LY, Wei L, Jing QK. Changes of motor function, Nt-3 and Trk C expression in rats at 14 day post-spinal cord injury and the effect of different electroacupuncture. Sci Sin Vitae 2016; 46: 983-989.
- Herrity AN, Petruska JC, Stirling DP. The effect of spinal cord injury on the neurochemical properties of vagal sensory neurons. Am J Physiol Regul Integr Comp Physiol 2015; 308: 1021-1033.
- Hosier H, Peterson D, Tsymbalyuk O. A direct comparison of three clinically relevant treatments in a rat model of cervical spinal cord injury. J Neurotrauma 2015; 32: 1633-1644.
- Zhang RP, Wang LJ, He S. Effects of magnetically guided, SPIO-labelled, and Neurotrophin-3 gene-modified bone mesenchymal stem cells in a rat model of Spinal Cord Injury. Stem Cells Intern 2016; 22: 1-11.
- Effects of Huoxue Tongdu decoction on the injury reaction of spinal cord after sciatic nerve injury. Chin J Tradit Med Traumatol Orthop 2016; 24: 1-3.
- Wu YP, Fan X, Zhang L. Effects of Huoxue Tongdu decoction on expression of nerve growth factor and glial fibrillary acidic protein in rats after acute spinal cord injury. Chin J Trad Chin Med Pharm 2017; 32: 697-701.
- Mosquera L, Colón JM, Santiago JM. Tamoxifen and estradiol improved locomotor function and increased spared tissue in rats after spinal cord injury: their antioxidant effect and role of estrogen receptor alpha. Brain Res 2014; 1561: 11-22.
- Ahn H, Bailey CS, Rivers CS. Effect of older age on treatment decisions and outcomes among patients with traumatic spinal cord injury. CMAJ 2015; 187: 873-880.
- Squair JW, le Nobel G, Noonan VK. Assessment of clinical adherence to the international autonomic standards following spinal cord injury. Spinal Cord 2015; 53: 668-672.
- Guilcher SJ, Parsons D, Craven BC. Developing quality of care indicators for patients with traumatic and non-traumatic spinal cord injury (SCI): A feasibility study using administrative health data. J Spinal Cord Med 2015; 38: 765-776.
- Soubeyrand M, Dubory A, Laemmel E. Effect of norepinephrine on spinal cord blood flow and parenchymal haemorrhage size in acute-phase experimental spinal cord injury. Eur Spine J 2014; 23: 658-665.
- Liu Q, Liu Q, Zhao J. Early MRI finding in adult spinal cord injury without radiologic abnormalities does not correlate with the neurological outcome: a retrospective study. Spinal Cord 2015; 53: 750-753.
- Tokmak M, Yuksel Y, Sehitoglu MH. The neuroprotective effect of syringic acid on spinal cord ischemia/reperfusion injury in rats. Inflamm 2015; 38: 1969-1978.
- Chen J, Zhang Z, Liu J. Cellular spinal cord scaffold seeded with bone marrow stromal cells protects tissue and promotes functional recovery in spinal cord-injured rats. J Neurosci Res 2014; 92: 307-317.
- Yu D, Li M, Ni B. Induction of neuronal mitophagy in acute spinal cord injury in rats. Neurotox Res 2013; 24: 512-522.
- Guven M, Sehitoglu MH, Yuksel Y. The neuroprotective effect of coumaric acid on spinal cord ischemia/reperfusion injury in rats. Inflamm 2015; 38: 1986-1995.
- Lin QB, Zhang L. Effect of Huoxue Tongdu decoction on the expressions of TNF-α, IL-1β and IL-8 in spinal cord ischemia-reperfusion injury of rabbits. Chin J Trad Chin Med 2015; 30: 2477-2480.
- Reyes-Alva HJ, Franco-Bourland RE, Martinez-Cruz A. Spatial and temporal morphological changes in the subarachnoid space after graded spinal cord contusion in the rat. J Neurotrauma 2013; 30: 1084-1091.
- Talbott JF, Whetstone WD, Readdy WJ. The brain and spinal injury center score: a novel, simple, and reproducible method for assessing the severity of acute cervical spinal cord injury with axial T2-weighted MRI findings. J Neurosurg Spine 2015; 23: 495-504.