Research Article - Biomedical Research (2017) Volume 28, Issue 2
The relationship between serum fetuin a levels and fetuin gene polymorphism in hemodialysis patients
Atila Altuntaş1*, Ayşe Yiğit2, Efkan Uz3, Salih İnal1, Veysel Kidir1, BünyaminAydin4, Hasan Basri Savaş3, Mehmet Sert5, Mehmet Tuğrul Sezer11Department of Internal Medicine, Division of Nephrology, Süleyman Demirel University School of Medicine, Isparta, Turkey
2Department of Medical Genetics, Süleyman Demirel University School of Medicine, Isparta, Turkey
3Department of Medical Biochemistry, Süleyman Demirel University School of Medicine, Isparta, Turkey
4Department of Internal Medicine, Division of Endocrinology and Metabolism, Süleyman Demirel University School of Medicine, Isparta, Turkey
5Division of Nephrology, Isparta State Hospital, Isparta, Turkey
- *Corresponding Author:
- Atila Altuntaş
Süleyman Demirel University School of Medicine
Department of Internal Medicine
Division of Nephrology, 32260, Cunur - Isparta, Turkey
E-mail: atilaaltuntas@yahoo.com
Accepted date: May 04, 2016
Abstract
Introduction: Fetuin A, also called Heramans Schmid alpha 2 glycoprotein (AHSG), is one of the important proteins that inhibit vascular calcification. In this study, we aimed to evaluate relationship between AHSG gene polymorphism and fetuin A levels.
Materials and methods: 152 patients receiving regular hemodialysis treatment and 61 healthy controls were included to this cross-sectional study. Serum fetuin-A levels were assessed by ELISA method. Thr256Ser and Thr248Met gene polymorphisms are determined by PCR-RFLP.
Results: Serum fetuin A level in hemodialysis patients (330.5 ± 171.2 mg/L) was significantly lower as compared to control group (382.9 ± 138.5 mg/L) (p=0.001). Significant negative correlation between fetuin-A and C-reactive protein (CRP) (r=-0.28, p<0.0001) was found. The distribution of Thr256Ser and Thr248Met gene polymorphisms in hemodialysis and control groups were similar. In hemodialysis group, serum fetiun A levels in the patients with genotype Thr/Thr (n=94, 366.9 ± 184.2 mg/L) were found to be singnificantly higher than in the patients with genotype Thr/Ser (n=52, 278.1 ± 132.7 mg/L) and Ser/Ser (n=6, 212.5 ± 63.3 mg/L) (respectively; p=0.005, p=0.022). Unlike Thr256Ser polymorphism, serum Fetuin-A levels did not differ between Thr248Met gene polymorphism genotypes.
Conclusion: The current study showed that HD patients with altered polymorphism of the AHSG Thr256Ser gene appear to be a negative prognostic factor on serum Fetuin-A levels. In other words, it can be speculated that fetuin-A Thr256Ser gene polymorphism, particularly genotypes Thr/Ser and Ser/ Ser, may be an additional promoting risk factor for vascular ossification in HD patients.
Keywords
Fetuin A, Genetic Polymorphism, Hemodialysis, Inflammation.
Introduction
The mortality rate among dialysis patients is obviously more than that of the general population and cardiovascular diseases are the leading causes of mortality among dialysis patients [1,2]. This very high cardiovascular mortality rate in this population is only partially explained by the high prevalence of traditional risk factors which are classically related to atherosclerosis [3]. The vascular changes observed in chronic kidney disease (CKD) are not only based on atherosclerosis, but also arteriosclerosis is widely seen associated with pathological vascular calcification of both media and intima [4]. Eventually, arterial stiffening and the extent of calcification are reported to be strong prognostic markers of mortality rates in hemodialysis (HD) patients [5]. It is assumed that this may be partially related to a relative lack of calcification inhibitors or vascular protective factors like fetuin-A.
Fetuin-A, also known as α2-Heremans-Schmid glycoprotein (AHSG), is a circulating negative acute-phase glycoprotein with 62-kDa molecular weight [6]. It is synthesized in liver and is a member of cystatin superfamily of cysteine proteases. Fetuin-A is involved in several biological processes including the inhibition of ectopic mineral calcification and decreased serum levels are reported during inflammation [7,8]. In vitro studies have demonstrated that, fetuin-A inhibits the apatite formation and that it significantly accounts for the ability to prevent mineral precipitation [9]. It binds to calcium phosphate with severe adherence and acts as a buffer for serum calcium phosphate. In animal models of CKD, fetuin-A inhibits pathological calcification in the vasculature, protecting against atherosclerotic plaque formation [10]. In accordance with this, massive ectopic calcification occurs in fetuin-A knockout mice receiving a diet with a high content of calcium and vitamin D [11]. The role of fetuin-A has also been studied in clinical trials. Lower serum fetuin-A concentrations have been shown to increase the risk of cardiovascular disease [12,13]. Moreover, fetuin-A levels are found significantly lower in CKD patients with calciphylaxis compared with other CKD subjects [11]. Based on these evidences, it is assumed that deficiency in fetuin-A likely contributes to vascular calcifications in CKD. There is also some evidence in the literature about the relation between serum levels and polymorphisms in the gene encoding fetuin-A [13,14]. However, the results are conflicting and in addition they are mainly based on AHSG T256S gene polymorphism. Therefore we aimed to investigate the relation between serum Fetuin-A levels and polymorphisms of AHSG T256S and T248M genes which are encoding Fetuin-A, in a Turkish HD patient population.
Materials and Methods
Patients and general characteristics
This cross-sectional, single-center study enrolled patients with end stage renal disease (ESRD) having HD treatment and agematched healthy control subjects. The patients in the HD group were recruited from the adult patients receiving regular dialysis treatment thrice a week for four hours with standard bicarbonate dialysate and with synthetic membranes for more than 9 months. Exclusion criteria were; history or existence of malignancy, chronic liver diseases, autoimmune diseases, symptoms of active infection or inflammation, hepatitis B and C virus infection and being on steroid treatment. 152 patients receiving regular HD treatment and 61 healthy controls were included to the final evaluation. The study protocol was approved by Local Medical Ethics Committee. It was performed in accordance with the principles of the Helsinki Declaration and informed consent was obtained from all participants. Demographic and clinical data were screened from medical records including age, gender, smoking habits, primary kidney disease, time on dialysis, medications, Kt/ Vurea (Single pool urea clearance index, used to represent weekly dialysis dose; where K is the clearance of urea, t is the dialysis time and V is the volume of distribution of urea) and comorbid conditions. Anthropometric measurements were performed in all subjects and body mass index (BMI) was calculated as the ratio of weight (kg) to the square of height (m). Arterial blood pressure was measured three times with an appropriate cuff after at least 10 minute resting and the average value was taken into account.
Biochemical and genetic analysis
For the determination of serum Fetuin A, calcium, phosphorus, C-reactive protein (CRP) and other serum parameter concentrations, venous blood samples were collected and after centrifugation at 3000g for 10 minutes (NF 800, Turkey), sera were stored at -80°C until the assay. Venous blood samples from HD patients were drawn in the morning of a mid-week dialysis session prior to heparinization. Venous blood samples were drawn from the control group after an over-night fasting period. Serum Fetuin-A levels were assessed by ELISA method (Epitope Diagnostics, Inc., San Diego, Calif., USA). AHSG (Fetuin-A) Thr256Ser and Thr248Met gene polymorphisms are determined by PCR-RFLP. Genomic DNA was extracted by use of the Wizard Genomic DNA purification Kit (Promega). Analysis of the Fetuin-A Thr248Met polymorphism was performed with the primers 5’- CCTCCCACAAGCAGAAAC -3’ and 5’- TGATGATTCCGCATACCC -3’. PCR was performed in a 50 mcL reaction volume containing approximately 65 ng of genomic DNA, 25 pmol of each primer (Biomers), 1.5 U of Taq DNA polymerase (Promega) in 1X Reaction Buffer supplied with the enzyme, and 0.25 mM of each deoxynucleotide triphosphate (Promega). The PCR conditions were as follows: initial denaturation at 95°C for 5 min; 35 cycles of denaturation at 95°C for 1 min, annealing at 56°C for 1 min, and extension at 72°C for 1 min; and a final extension at 72°C for 7 min.
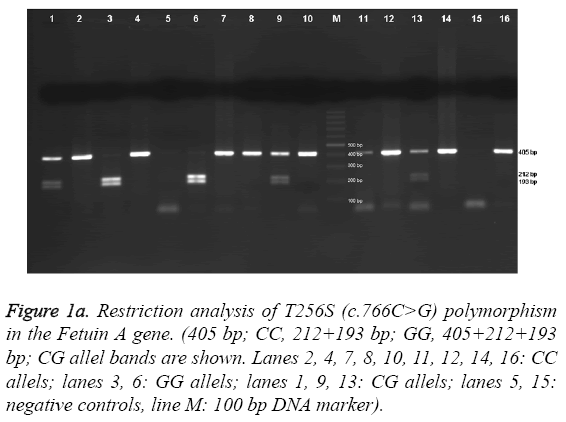
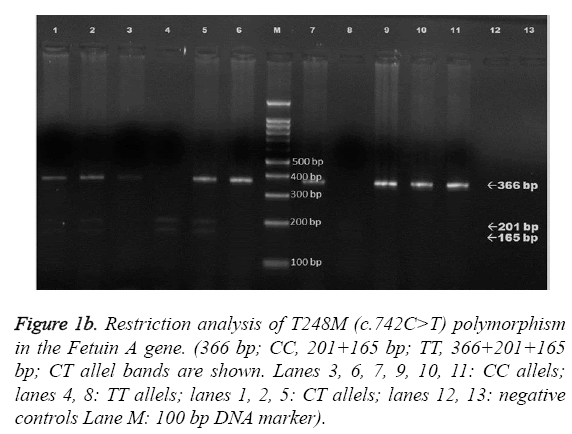
The obtained 366-bp fragments were digested at 37 °C with 2.5 U of NlaIII overnight and separated on a 2 % agarose gel. The Fetuin-A Thr256Ser polymorphism was analyzed with use of the primers 5’-GTCACCCCTCCTTGTAAC-3’ and 5’- CCCCAATGAGACCACA-3’. The reaction mixture and PCR conditions were as described above. The amplified 405-bp products were digested at 37 °C with 5 U of SacI (restriction enzyme) overnight and separated on a 1.5 % agarose gel. Electrophoresis images of AHSG Thr256Ser and Thr248Met gene polymorphisms are shown in Figure 1a and Figure 1b, respectively. For T256S, the c.766C allele remained undigested, whereas the c.766G allele yielded 193- and 212-bp fragments. For T248M, the c.742T allele yielded 165- and 201- bp fragments, whereas the c.742C allele remained undigested.
Figure 1a: Restriction analysis of T256S (c.766C>G) polymorphism in the Fetuin A gene. (405 bp; CC, 212+193 bp; GG, 405+212+193 bp; CG allel bands are shown. Lanes 2, 4, 7, 8, 10, 11, 12, 14, 16: CC allels; lanes 3, 6: GG allels; lanes 1, 9, 13: CG allels; lanes 5, 15: negative controls, line M: 100 bp DNA marker).
Figure 1b: Restriction analysis of T248M (c.742C>T) polymorphism in the Fetuin A gene. (366 bp; CC, 201+165 bp; TT, 366+201+165 bp; CT allel bands are shown. Lanes 3, 6, 7, 9, 10, 11: CC allels; lanes 4, 8: TT allels; lanes 1, 2, 5: CT allels; lanes 12, 13: negative controls Lane M: 100 bp DNA marker).
Statistical analysis
All continuous variables were tested for normality of distribution by using Kolmogorov-Smirnov test. Log transformation was performed for non-normally distributed variables. Continuous data were presented as mean ± standard deviation (SD). Categorical variables were compared using chi-squared test and shown as frequency and percentages. Comparisons of parametric data of three groups were performed with One-Way ANOVA test. Levene’s test was used to determine homogeneity of variances. Post hoc Tukey test (in case of homogeneity of variance) or Dunnett’s t3 test (in case of heterogeneity of variance) was used. Three group comparisons for non-parametric data were performed by Kruskal Wallis-test with post hoc Bonferroni Correction test; in this test statistical significance was accepted when p<0.016. Spearman or Pearson rank correlation tests were performed to determine the relationship between continuous variables where appropriate. The potential predictor factors of elevated Fetuin A level were evaluated using a multivariate linear regression analysis model based on significant correlations. Statistical analysis was performed using SPSS software (version 15.0 SPSS; Chicago, IL, USA). All p-values were calculated as twosided, and p-value<0.05 was considered as significant.
Results
A total of 152 HD patients with mean age of 62.0 ± 13.6 and 61 healthy subjects with mean age of 60.5 ± 10.3 years were enrolled into the study. 53 and 49 percent of study population were males in HD and control groups, respectively (p=0.58). Besides gender distribution, HD patients and healthy controls were also similar in terms of age (62.0 ± 13.6 vs. 60.5 ± 10.3, p=0.37) and BMI (24.4 ± 4.0 vs. 25.1 ± 4.3, p=0.66). Serum Fetuin-A level (330.5 ± 171.2 vs. 382.9 ± 138.5 mg/L, p=0.001) was significantly lower and serum CRP level (12.4 ± 14.1 vs. 6.2 ± 6.6, p=0.004) was significantly higher in HD patients compared to control group. Demographic, clinical and laboratory findings of two groups are shown in Table 1.
| HD (n=152) | Control (n=61) | P value | |
|---|---|---|---|
| Age, years | 62±13.6 | 60.5±10.3 | 0.375 |
| Males, % | 53.3 | 49.2 | 0.587 |
| BMI (kg/m2) | 24.4±4.05 | 25.12±4.31 | 0.662 |
| SBP (mm/hg) | 132.4±16.8 | 118±8.5 | <0.001 |
| DBP (mm/Hg) | 80.9±9.2 | 75.8±5.3 | <0.001 |
| Smoking (%) | 14.1 | 11.3 | 0.610 |
| Calcium (mg/dl) | 8.73±1 | 9.18±0.69 | <0.001 |
| Phosphorus (mg/dl) | 4.97±1.33 | 3.01±0.53 | <0.001 |
| CaxP product (mg2/dl2) | 43.47±12.45 | 27.63±5.34 | <0.001 |
| Parathyroid hormone (pg/ml) | 425.6±388.2 | - | - |
| S-albumin (g/dl) | 4.06±0.4 | 4.05±0.37 | 0.570 |
| S-LDL (mg/dl) | 94.3±34.86 | 125±28.21 | <0.001 |
| CRP (mg/L ) | 12.44±14.11 | 6.21±6.65 | 0.004 |
| BUN (mg/dl) | 59.7±19.4 | 14.12±4.79 | <0.001 |
| Creatinine(mg/dl) | 7.56±2.89 | 0.9±0.19 | <0.001 |
| Fetuin A (mg/L) | 330.5±171.2 | 382.9±138.5 | 0.001 |
| Cardiovascular events (%) (stroke, coronary events) | 66.4 | - | - |
| Duration of HD, months | 50.2±39.6 | - | - |
| Kt/V | 1.64±0.55 | - | - |
| Hemoglobin(g/dl) | 11.8±7.24 | - | - |
| Transferrin saturation (%) | 38.8±23.7 | - | - |
| Ferritin (ng/ml) | 992.5±590.1 | - | - |
| The etiology of CKD (%) | - | - | |
| Diabetes mellitus | 35.8 | - | - |
| Hypertension | 28.5 | - | - |
| Glomerulonephritis | 6.6 | - | - |
| Polycystic kidney disease | 6.6 | - | - |
| Nephrolithiasis | 5.3 | - | - |
| Amyloidosis | 3.3 | - | - |
| Etiology unknown | 9.3 | - | - |
| Other | 4.6 | - | - |
Table 1: Demographic, clinical and laboratory findings of hemodialysis and control groups.
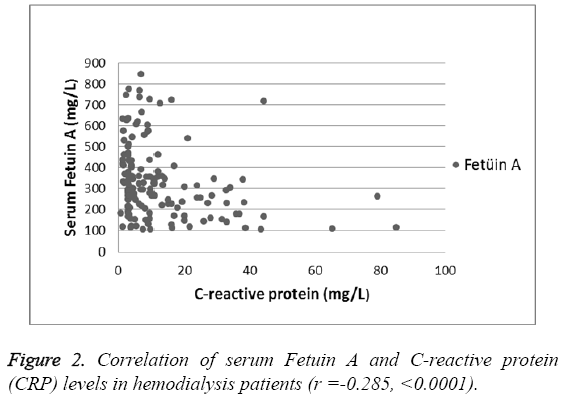
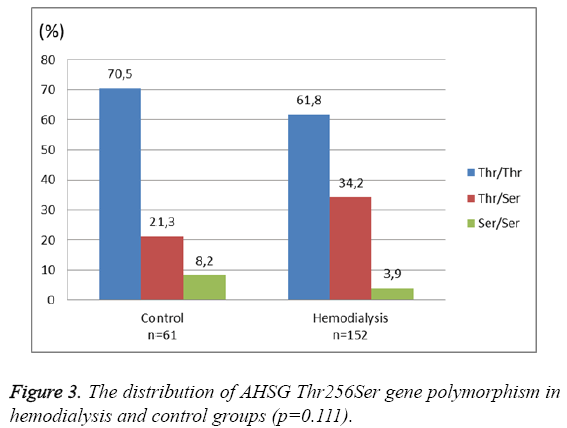
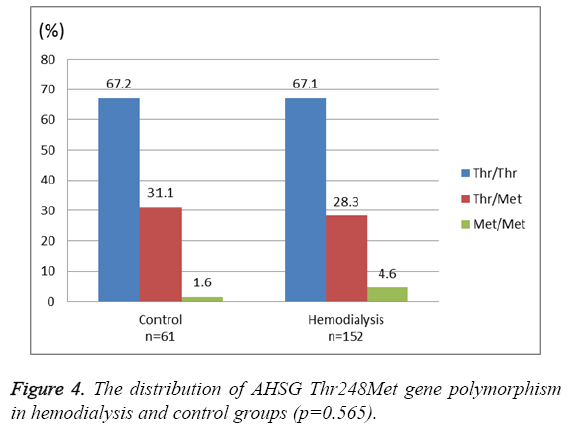
There was a significant negative correlation between Serum Fetuin-A levels and CRP (Pearson's r=-0.28, p<0.0001) (Figure 2). However, no significant association was found between serum Fetuin-A levels and albumin levels, dialysis duration, parathyroid hormone (PTH), low-density lipoprotein (LDL) and calcium–phosphorous product (p>0.05, for all). As shown in Figure 3 and Figure 4, the distribution of AHSG Thr256Ser and Thr248Met gene polymorphisms in HD and control groups were similar (p=0.11 and 0.56, respectively).
In HD group, serum Fetuin-A levels were assessed between genotype subgroups of both AHSG Thr256Ser and Thr248Met gene polymorphisms. Among the three Thr256Ser genotypes, serum Fetuin-A levels in the patients with genotype Thr/Thr (n=94, 366.9 ± 184.2 mg/L) were found to be significantly higher than in the patients subgroup with genotype Thr/Ser (n=52, 278.1 ± 132.7 mg/L) and Ser/Ser (n=6, 212.5 ± 63.3 mg/L) (p=0.005 and p=0.022, respectively). Serum Fetuin-A levels in the patients with genotype Thr/Ser and Ser/Ser were similar (p=0.211). Demographic, clinical and laboratory findings of hemodialysis and control groups according to Thr256Ser gene polymorphism are shown in Table 2. Unlike Thr256Ser polymorphism, serum Fetuin-A levels did not differ between Thr248Met gene polymorphism genotypes (Table 3).
| Thr/Thr n=94 |
Thr/Ser n=52 |
Ser/Ser n=6 |
P value | |
|---|---|---|---|---|
| Hemodialysis Group | ||||
| Age, years | 61.4±13.2 | 62.7±14.7 | 65.2±10.9 | 0.726 |
| Males, % | %53.2 | %50 | %83.3 | 0.301 |
| BMI (kg/m2) | 24.7± 4 | 23.7±4 | 25.1±4.8 | 0.390 |
| SBP (mm/hg) | 130.6±15.4 | 134.9±18.7 | 138.3±19.4 | 0.339 |
| DBP (mm/Hg) | 81±9.8 | 80.8±8.4 | 81.7±7.5 | 0.940 |
| Smoking (%) | %14.1 | %13.7 | %16.7 | 0.981 |
| CaxP product (mg2/dl2) | 42.1±11.9 | 46.4±13.2 | 39.5±10.2 | 0.095 |
| Parathyroid hormone (pg/ml) |
408±408 | 453.7±361.4 | 456.4±327.5 | 0.339 |
| S-albumin (g/dl) | 4.04±0.38 | 4.1±0.44 | 4.1±0.35 | 0.533 |
| S-LDL (mg/dl) | 94.1±34.4 | 93.8±35.4 | 102.8±42.3 | 0.832 |
| CRP ( mg/L ) | 13.2±15.4 | 10.8±11.4 | 14.3±15.9 | 0.842 |
| BUN (mg/dl) | 59.4±17.4 | 59.8±23 | 63.9±17 | 0.669 |
| Creatinine(mg/dl) | 7.5±3.2 | 7.5±2.4 | 8.2±1.4 | 0.434 |
| Fetuin A (mg/L) | 366.9 ±184.2a | 278.1±132.7b | 212.5±63.3b | 0.003 |
| Cardiovascular events(%) (stroke, coronary events) |
35.9 | 27.9 | 50 | 0.406 |
| Duration of HD, months | 48.3±36.2 | 49.4±41.8 | 86.7±58.5 | 0.178 |
| Kt/V | 1.66±0.67 | 1.63±0.3 | 1.35±0.23 | 0.065 |
| Drugs used (%) | ||||
| Calcium acetate | 58.5 | 65.4 | 66.7 | 0.689 |
| Sevelamer | 26.6 | 40.4 | 33.3 | 0.228 |
| Calcitriol | 25.5 | 15.4 | 16.7 | 0.346 |
| Paricalcitol | 26.6 | 28.8 | 33.3 | 0.910 |
| Cinacalcet | 10.6 | 19.2 | 33.3 | 0.150 |
| Cholecalciferol | 1.1 | 3.8 | 16.7 | 0.055 |
| ACEİ | 19.1 | 11.5 | 4 | 0.494 |
| ARB | 8.5 | 11.5 | 0 | 0.606 |
| CCB | 23.3 | 11.5 | 0 | 0.134 |
| BB | 41.5 | 30.8 | 66.7 | 0.160 |
| Statins | 8.5 | 11.5 | 33.3 | 0.151 |
| Thr/Thr n=43 |
Thr/Ser n=13 |
Ser/Ser n=5 |
P value | |
| Control Group | ||||
| Age, years | 61±9.9 | 58.5±12 | 61.2±9.5 | 0.726 |
| Males, % | 55.8 | 30.8 | 40 | 0.261 |
| BMI(kg/m2) | 25.2±4.6 | 24.4±1.4 | 26.1±7.1 | 0.785 |
| Smoking (%) | 7.7 | 22.2 | 20 | 0.377 |
| S-albumin (g/dl) | 4.03±0.32 | 4.18±0.41 | 3.77±0.61 | 0.132 |
| CRP | 6.7±7.5 | 3.5±1 | 9.6±6.6 | 0.120 |
| Fetuin A (mg/L) | 395.7±146 | 368.8±131.4 | 309.6±53.2 | 0.392 |
| Hypertension (%) | 18.6 | 7.7 | 40 | 0.275 |
Table 2: Demographic, clinical and laboratory findings of hemodialysis (n=152) and control (n=61) groups according to Thr256Ser gene polymorphism.
| Thr/Thr n=102 |
Thr/Met n=43 |
Met/Met n=7 |
P value | |
|---|---|---|---|---|
| HemodialysisGroup | ||||
| Age, years | 61.6±13.1 | 62.5±15.3 | 64.6±13.6 | 0.837 |
| Males, % | 54.9 | 44.2 | 85.7 | 0.106 |
| BMI (kg/m2) | 24.1±3.9 | 25.1±4.4 | 24.8±4.1 | 0.467 |
| SBP (mm/hg) | 130.2±15.6 | 136.7±18.7 | 137.1±18 | 0.088 |
| DBP (mm/Hg) | 81.3±9.8 | 80±7.9 | 80.7±7.3 | 0.594 |
| Smoking (%) | 16.2 | 7 | 28.6 | 0.186 |
| CaxP product (mg2/dl2) | 43.5±13.2 | 43.7±11.2 | 41.5±10.6 | 0.906 |
| Parathyroid hormone (pg/ml) |
407,4±405 | 470.6±360.4 | 414.2±319.5 | 0.267 |
| S-albumin (g/dl) | 4.06±0.36 | 4.05±0.47 | 4.2±0.35 | 0.669 |
| S-LDL (mg/dl) | 93.5±34.2 | 95.8±36 | 96.7±41.9 | 0.926 |
| CRP ( mg/L ) | 13.2±15.2 | 10.4±11.1 | 14.2±14.5 | 0.909 |
| BUN (mg/dl) | 59.8±18.5 | 59.2±22.1 | 61.7±16.6 | 0.946 |
| Creatinine(mg/dl) | 7.5±3.2 | 7.5±2.3 | 8.2±1.3 | 0.379 |
| Fetuin A (mg/L) | 350.4 ±177.2 | 299.3±159.5 | 231.6±76.9 | 0.069 |
| Cardiovascular events (%) (stroke, coronary events) |
35.4 | 25.6 | 57.1 | 0.210 |
| Duration of HD, months | 49.7±38.3 | 45.7±38.4 | 84.6±53.7 | 0.096 |
| Kt/V | 1.66±0.64 | 1.65±0.3 | 1.37±0.22 | 0.073 |
| Drugs used (%) | ||||
| Calcium acetate | 56.8 | 69.8 | 71.4 | 0.294 |
| Sevelamer | 30.4 | 34.9 | 28.6 | 0.855 |
| Calcitriol | 23.5 | 18.6 | 14.3 | 0.715 |
| Paricalcitol | 25.5 | 30.2 | 42.9 | 0.551 |
| Cinacalcet | 12.7 | 16.3 | 28.6 | 0.476 |
| Cholecalciferol | 1 | 4.7 | 14.3 | 0.065 |
| ACEİ | 18.6 | 11.6 | 14.3 | 0.576 |
| ARB | 8.8 | 9.3 | 14.3 | 0.889 |
| CCB | 16.7 | 20.9 | 14.3 | 0.804 |
| BB | 41.2 | 30.2 | 57.1 | 0.278 |
| Statins | 7.8 | 14 | 28.6 | 0.154 |
| Thr/Thr n=41 |
Thr/Met n=19 |
Met/Met n=1 |
P value | |
| Control Group | ||||
| Age, years | 59.9±10.5 | 61.5±10.1 | 67 | 0.697 |
| Males, % | 53.7 | 42.1 | 0 | 0.432 |
| BMI(kg/m2) | 24.5±3.2 | 25.7±5.4 | 38 | 0.228 |
| Smoking (%) | 7.3 | 20 | 0 | 0.442 |
| S-albumin (g/dl) | 4.03±0.37 | 4.09±0.39 | 4.1 | 0.842 |
| CRP | 6.7±7.8 | 5.3±3.1 | 5 | 0.701 |
| Fetuin A (mg/L) | 397.1±136.9 | 359.5±142.1 | 245.4 | 0.382 |
| Hypertension (%) | 17.1 | 15.8 | 100 | 0.098 |
Table 3: Demographic, clinical and laboratory findings of hemodialysis (n=152) and control (n=61) groups according to Thr248Met gene polymorphism.
In order to find the predictor factors of elevated Fetuin A level, a multivariate linear regression analysis model was established. Age, dialysis duration, gender, serum albumin and CRP level, Tre256Ser and Thr248Met polymorphisms were taken as possible predictors on serum Fetuin A level. Stepwise multiple regression analysis model revealed independent relationship between AHSG Thr256Ser gene polymorphism (β:-0.271, p<0.0001, 95% CI: -0.403, -0.14), CRP (β:-0.013, p<0.0001, 95% CI:-0.018, -0.008) and Fetuin-A level (log-transformed) in HD group, after correcting for the impact of age, gender, serum albumin and dialysis duration.
Discussion
In the present study, we evaluated the potential effects of AHSG T256S and T248M gene polymorphisms on serum fetuin-A levels in a Turkish HD patients group. Our findings revealed that, although T248M gene polymorphism seems not to be related with serum fetuin-A, T256S gene polymorphism and serum CRP levels are found to be independent predictors of serum fetuin-A levels in HD patients. Among T256S genotypes, Thr/Ser and Ser/Ser subgroups had significantly lower levels of Fetuin-A when compared with Thr/Thr genotype subgroup. Secondly, serum fetuin-A levels were significantly lower in HD patients compared with healthy controls and there was a significantly negative correlation between serum CRP and Fetuin-A levels.
The patients with advanced CKD usually have a generalized vascular ossification process that is an important predictor of death. There is substantial evidence indicating that fetuin-A is an important inhibitor of ectopic calcification [11,15]. Consistently, it is demonstrated that deficiency in fetuin-A is contributing to this vascular ossification process. In addition, fetuin-A is a negative acute phase reactant [16]. As the CKD patients, particularly patients on HD, are affected by a chronic inflammatory state, they are expected to have lower levels of serum fetuin-A. In some recent clinical studies, besides lower fetuin-A levels in HD patients, a reverse association was also observed between fetuin-A and CRP levels [12,17-19]. Our study has similarly revealed that serum fetuin-A levels were significantly lower than healthy controls and CRP levels were significantly higher than healthy controls. In addition, there was a negative correlation between fetuin-A levels and CRP. However, we could not demonstrate a correlation between fetuin-A and serum albumin levels unlike the previous reports [17,18]. Patients with low albumin levels were excluded and the mean albumin level of HD patients was 4.06 g/dl in our study. So, we assume that they were relatively well-nourished and this point probably cleared off the anticipated association between fetuin-A and albumin.
It has been demonstrated that serum Fetuin A levels are influenced by some AHSG gene polymorphisms both in vitro and in vivo [13,15,20]. The most known allelic variant in clinical studies is the T256S. Several allelic variants of this gene, especially the T256S substitution, have been shown to be associated with reduced serum levels of the protein [21]. Stenvinkel et al. showed that a low fetuin-A level was associated with malnutrition, inflammation, atherosclerotic plaques and ESRD patients with 256Ser allele were at a higher risk for accelerated vascular calcification [13]. Moreover, this polymorphism was reported to be associated with all-cause and cardiovascular mortality in ESRD patients [13].
However, the results about the impact of allelic variations on serum fetuin-A level and clinical outcomes are conflicting. In a study conducted by Cozzolini et al. serum fetuin-A level in Italian HD cohort was not associated with an alteration in the distribution of AHSG T256S polymorphisms [14]. In another study, although serum fetuin-A levels were higher in patients with pseudoxanthoma elasticum disease, which is a heritable disorder of the connective tissue, the frequencies of the T248M and T256S polymorphisms did not differ between the patients and healthy controls [22].
Our study is unique by studying both T248M and T256S polymorphisms in HD patients and additionally this is the first study on Turkish HD patients. According to our results, serum Fetuin-A levels did not differ between Thr248Met gene polymorphism genotypes. But T256S gene polymorphism was found to be an independent predictor of serum fetuin-A level and Thr/Ser and Ser/Ser subgroups had significantly lower levels of fetuin-A when compared with Thr/Thr genotype subgroup. So our results confirm the previous findings reported by Stenvinkel et al. [13]. Based on these findings, we suggest that CKD patients receiving HD treatment have already a reduction in serum fetuin-A levels and moreover lower levels of fetuin-A seems to be associated with alteration in the distribution of AHSG T256S polymorphisms.
A chronic inflammatory state and malnutrition can probably be counted among the major causes of low fetuin level in CKD patients, especially for ESRD patients on HD. Therefore, it seems that attempts on treatments for increasing fetuin-A levels and eliminating malnutrition in the HD patients may be helpful in some degree in preventing pathological calcification process. From this point of view, we suggest that clinicians engaged in CKD and dialysis treatments should be more attentive for patients with Thr/Ser and Ser/Ser genotypes of AHSG T256S polymorphisms in order to prevent excess vascular calcification. Perhaps measures like avoiding calcium based phosphate binders or setting lower dialysate calcium will be practical clinical implications for this more risky group if this evidence can be confirmed with further studies.
The present study has several limitations. First limitation is somewhat small number of the study population. A considerable number of HD patients have been excluded due to active infections, inflammatory disorders or severe malnutrition which could have potential effects on serum fetuin-A levels. Secondly, we did not demonstrate the effect of genetic polymorphism on vascular calcification and we did not put forward any clinical outcome of these allelic variations. Thirdly, our study has a cross sectional design and it lacks long term clinical follow-up.
In conclusion, the current study showed that HD patients with altered polymorphism of the AHSG T256S gene appear to be a negative prognostic factor on serum Fetuin-A levels. In other words, it can be speculated that fetuin-A Thr256Ser gene polymorphism, particularly genotypes Thr/Ser and Ser/Ser, may be an additional promoting risk factor for vascular ossification in HD patients. More investigations are required to elucidate the role of AHSG gene polymorphisms on Fetuin-A serum levels and particularly on clinical outcomes in HD patients.
Acknowledgement
This study was carried out with the support of Suleyman Demirel University Scientific Research Projects Coordination Unit.
Declaration of Interest
The authors have no financial disclosures to declare and no conflicts of interest to report. The authors are responsible for the content and writing of the paper.
References
- Bellomo G, Lippi G, Saronio P, Reboldi G, Verdura C, Timio F, Timio M. Inflammation, infection and cardiovascular events in chronic hemodialysis patients: a prospective study. J Nephrol. 2003; 16: 245-251.
- Nube MJ. The acute phase response in chronic hemodialysis patients: a marker of cardiovascular disease? Nephrol Dial Transplant 2002; 17: 19-23.
- Longenecker JC, Coresh J, Powe NR, Levey AS, Fink NE, Martin A, Klag MJ. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: the CHOICE study. J Am SocNephrol 2002; 13: 1918–1927.
- London GM. Cardiovascular calcifications in uremic patients: clinical impact on cardiovascular function. J Am SocNephrol 2003; 14: 305–309.
- Guérin AP, London GM, Marchais SJ, Metivier F. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant 2000; 15: 1014-1021.
- Denecke B, Graber S, Schafer C, Heiss A, Woltje M, Jahnen-Dechent W. Tissue distribution and activity testing suggest a similar but not identical function of fetuinB and fetuin-A. Biochem J 2003; 376: 135-145.
- London GM, Guerin AP, Marchais SJ, Métivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 2003; 18: 1731-1740.
- Welsh P, Doolin O, McConnachie A, Boulton E, McNeil G, Macdonald H, HardcastleA, Hart C, Upton M, Watt G, Sattar N. Circulating 25OHD, Dietary Vitamin D, PTH, and Calcium Associations with Incident Cardiovascular Disease and Mortality: The MIDSPAN Family Study. J ClinEndocrinolMetab 2012; 97: 4578-4587.
- Schinke T, Amendt C, Trindl A, Pöschke O, Müller-Esterl W, Jahnen-Dechent W. The serum protein alpha2-HS glycoprotein/fetuin inhibits apatite formation in vitro and in mineralizing calvaria cells: a possible role in mineralization and calcium homeostasis. J BiolChem 1996; 271: 20789–20796.
- Westenfeld R, Schafer C, Kruger T, Haarmann C, Schurgers LJ, Reutelingsperger C, Ivanovski O, Drueke T, Massy ZA, Ketteler M, Floege J, Jahnen-Dechent W. Fetuin-A protects against atherosclerotic calcification in CKD. J Am SocNephrol 2009; 20: 1264–1274.
- Schafer C, Heiss A, Shwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T, Jahnen-Dechent W. The serum protein α2-Heremans–Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J ClinInv 2003; 112: 357–366.
- Ketteler M, Bongartz P, Westenfeld R, Wildberger JE, Mahnken AH, Bohm R, Metzger T, Wanner C, Jahnen-Dechent W, Floege J. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet 2003; 361: 827–833.
- Stenvinkel P, Wang K, Qureshi AR, Axelsson J, Pecoits-Filho R, Gao P, Barany P, Lindholm B, Jogestrand T, Heimbürger O, Holmes C, Schalling M, Nordfors L. Low fetuin-A levels are associated with cardiovascular death: impact of variations in the gene encoding fetuin. Kidney Int 2005; 67: 2383-2392.
- Cozzolino M, Biondi ML, Galassi A, Gallieni M, d'Eril GV, Brancaccio D. Gene polymorphisms and serum alpha-2-Heremans-Schmid levels in Italian haemodialysis patients. Am J Nephrol. 2007; 27 : 639-642.
- Osawa M, Tian W, Horiuchi H, Kaneko M, Umetsu K. Association of alpha-2-HS glycoprotein (AHSG, fetuin-A) polymorphism with AHSG and phosphate serum levels. Hum Genet 2005; 116: 146–151.
- Lebreton JP, Joisel F, Raoult JP, Lannuzel B, Rogez JP, Humbert G. Serum concentration of human alpha-2 HS glycoprotein during the inflammatory process: evidence that alpha-2 HS glycoprotein is a negative acute phase reactant. J Clin Invest 1979; 64: 1118-1129.
- Haddad M, Tajbakhsh R, Farajollahi M, Qorbani M, Besharat S, Joshaghani HR. Association of serum fetuin-A and biochemical parameters in hemodialysis patients. Saudi J Kidney Dis Transpl. 2014; 25: 769-773.
- Wang AY, Woo J, Lam CW, Wang M, Chan IH, Gao P, Lui SF, Li PK, Sanderson JE. Associations of serum fetuin-A with malnutrition, inflammation, atherosclerosis and valvular calcification syndrome and outcome in peritoneal dialysis patients. Nephrol Dial Transplant 2005; 20: 1676-1685.
- Kim HR, Kim SH, Han MJ, Yoon YS, Oh DJ. The ratio of osteoprotegerin to fetuin-a is independently associated with vascular stiffness in hemodialysis patients. Nephron ClinPract. 2013; 123: 165-172.
- Bellia C, Tomaiuolo R, Caruso A, Sasso BL, Zarrilli F, Carru C, Deiana M, Zinellu A, Pinna S, Castaldo G, Deiana L, Ciaccio M. Fetuin-A serum levels are not correlated to kidney function in long-lived subjects. ClinBiochem 2012; 45: 637-640.
- OsawaM, Yuasa I, Kitano T, Henke J, KanekoM, Udono T, Saitou N, Umetsu K. Haplotype analysis of the human alpha2-HS glycoprotein (fetuin) gene. Ann Hum Genet 2001; 65: 27–34.
- Hendig D, Schulz V, Arndt M, Szliska C, Kleesiek K, Götting C. Role of serum fetuin-A, a major inhibitor of systemic calcification, in pseudoxanthomaelasticum. Clin Chem. 2006; 52: 227-234.