- Biomedical Research (2015) Volume 26, Issue 3
The relationship between HDL-associated PON1 activity, oxidative stress and brain natriuretic peptide in NYHA functional class ii-iv heart failure patients.
Esin Eren1*, Hamit Yasar Ellidag2, Ozgur Aydin3, Selcuk Kucukseymen4, Ozlem Giray2, Sakir Aslan4, Necat Yilmaz21Department of Biochemistry, Atatürk Hospital, Antalya, Turkey
2Central Laboratories of Antalya Education and Research Hospital of Ministry of Health, Antalya, Turkey
3Biochemistry Laboratory, Maternity and Children’s Hospital, Batman, Turkey
4Clinic of Cardiology, Antalya Education and Research Hospital of Ministry of Health, Antalya, Turkey
- *Corresponding Author:
- Esin EREN
Antalya Public Health Center of Ministry of Health
Clinical Biochemistry Laboratory
Antalya Egitim ve Arastirma Hastanesi
Varlik Mahallesi Kazim Karabekir Caddesi Soguksu
7100 Antalya, Turkey
Phone: 00902422494400
E-mail: esinerendr@gmail.com
Accepted date: February 19 2015
Abstract
As the role of oxidative stress on human diseases, including the cardiovascular system disorders, has accentuated, the ultimate need of reliable markers of oxidative stress becomes more imperative. We aimed to measure: activities of HDL-associated antioxidant enzymes, paraoxonase (PON1) and arylesterase (ARE); total oxidative (TOS) and antioxidative status (TAS), in heart failure (HF) patients, and search for correlations of these markers. The study group consisted of 70 subjects with HF. The patients were classified in three groups according to the suggestions of New York Heart Association. Serum levels of PON1, ARE, TOS, TAS, brain natriuretic peptide (BNP), uric acid (UA), creatinine, and lipid parameters were determined. The oxidative status index (OSI) was calculated. Plasma PON1 activity was significantly decreased (p=0.04), while BNP and UA levels were significantly increased(p<0.0001, p= 0.03, respectively), with the severity of the disease. ARE, TAS, TOS, and OSI did not show any statistically significant difference. Statistical analysis showed negative correlation between PON1, ARE activities and BNP; positive correlation between disease duration, UA and BNP. Also, a positive correlation was determined between TAS and UA. We report for the first time a notable relationship between HDL-associated anti-oxidant PON-1 activity and New York Heart Association classification for HF .These prominent results provide further support for the role for oxidative processes in the disease progression of HF and for the anti-oxidant compensatory role of HDL. We believe in the potential of antioxidant medications in HF and promote proper oxidative stress markers in routine use in diagnosis and follow-up.
Keywords
heart failure, oxidant, antioxidant, paraoxonase, arylesterase, oxidative stress, brain natriuretic peptide
Introduction
Heart failure (HF) is defined as a complex clinical syndrome that can result from any structural or functional cardiac disorder that impairs the ability of the ventricle to fill with or eject blood. It is a progressive disorder that must be managed with regard to, not only the state of the heart, but the condition of the circulation, lungs, neuroendocrine system and other organs as well. The major causes of HF are myocardial infarction, hypertension, cardiomyopathy and valvular heart disease [1]. Its prevalence is between 0.8% and 2% in the general population. HF is a leading cause of morbidity and mortality in industrialized countries. The prevalence of heart failure approximately doubles with each decade of life. As people live longer, the incidence of heart failure rises, along with other conditions which further complicate its treatment [2].
The high prevalence of cardiovascular diseases has made oxidative stress an ongoing focus of medical research. Its role in the development of cardiovascular injuries has been reliably established [3]. Over the past few decades, clinical and experimental studies have provided substantial evidence that oxidative stress, defined as an excess production of reactive oxygen species (ROS) relative to antioxidant defense, is enhanced in HF (Oxidative stress represents an imbalance between free radical production and endogenous antioxidant defenses and is known to be elevated in HF). Indeed, an increased concentration of free radicals, particularly superoxide, have been linked to peripheral hypoperfusion, peripheral endothelial dysfunction, and exaggerated sympathetic nerve activity in patients with HF. Recently, oxidant stress has been reported as being involved in the pathogenesis of HF [4,5].
There is substantial evidence that oxidative stress can activate many cellular responses that are characteristic of what occurs in heart failure, including cellular hypertrophy, changes in gene expression, and cell death. Classic stimuli for ventricular remodeling including wall stress, inflammatory cytokines and neurohormones including catecholamines and angiotensin II all appear to induce cellular changes at least in part, via oxidative or nitrosative stress. The pathways for activation of cellular phenotypes of hypertrophy and apoptosis appear to involve one or more stress-responsive protein kinases, many of which are activated by ROS [6].
Normal functional HDL has high levels of anti-oxidants, active anti-oxidant proteins and enzymes with high antioxidant potential and anti-inflammatory activity. Many pathological processes associated with systemic inflammation are characterized by the presence of dysfunctional or pro-inflammatory HDL, including HF, cardiovascular disease, migraine, metabolic syndrome, chronic kidney disease, infections and some rheumatological diseases [7].
Multifunctional enzyme paraoxonase (PON1) is a HDLassociated esterase/lactonase, which derived its name from one of its most commonly used in vitro substrates, paraoxon. Chronic pathological conditions associated with oxidative stress, such as HF, chronic renal failure, rheumatoid arthritis, migraine and Alzheimer’s disease, are frequently associated with reduced activity of PON1 enzyme activities [8-10]. PON1 exerts a protective effect against oxidative damage of cells and lipoproteins and modulates the susceptibility of HDL and LDL to atherogenic modifications such as homocysteinylation [9]. There are two polymorphisms in the PON1 coding region at positions Gln192→Arg (Q192R) and Leu55→Met (L55M). Paraoxon is hydrolysed six times faster by PON1 192R than by PON1 192Q, whereas the Q form is more active towards sarin, soman and diazoxon. The Q192R polymorphism also alters the enzyme’s ability to protect LDL from oxidation in vitro, the Q form being the most protective [9]. The precise pathophysiologic mechanism of PON-1 activity in human HF has not been previously examined.
European Guidelines (2008) emphasized the role of natriuretic peptides as potential markers for HF. Although most studies showed that brain natriureticpeptide (BNP) is a marker with a higher sensitivity and specificity; the application of this analysis in clinical practice is often limited by the absence of a universally accepted normal range. BNP is a hormone that is secreted predominantly by the ventricles, and reaches very high plasma concentrations in subjects with congestive HF. BNP is synthesized in the heart as a reaction to cardiac wall distension and stretching and neurohormonal activation [11].
In this study we measured: the serum activity of PON1 and arlyesterase (ARE), the total antioxidant status (TAS), the total oxidative status(TOS), oxidative stress index (OSI) and routine cardiac parameters, in patients with systolic chronic HF. Here we sought to determine the anti-oxidative role of serum HDL associated PON1 activity in subjects with HF, particularly in relation to established HF biomarkers.
Materials and Methods
Study population
The present analysis included 70 consecutive subjects with systolic heart failure (left ventricular ejection fraction [LVEF] < 45%) as determined by echocardiography. The patients included were suffering from clinical heart failure as diagnosed by the treating HF specialist in the cardiology center. Patients were classified according to the suggestions of New York Heart Association, as class II, III, and IV [12]. In total, 18 patients in class II, 32 patients in class III, and 20 patients in class IV, were enrolled as the study group.
Major exclusion criteria were: (i) chronic heart failure with preserved left ventricular ejection fraction (LVEF>45%); (ii) acute heart failure, acute myocardial infarction and myocarditis; (ii) known current or past allergic diseases, autoimmune diseases, inflammatory diseases or malignant diseases. Smokers and alcohol users were also excluded.
This study was performed in accordance with the ethical standards set by the Declaration of Helsinki and was approved by the local ethics committee.
Analytical methods
BNP assay
BNP assay is a fully automated (ADVIA Centaur) twosite sandwich immunoassay using direct chemiluminescent technology, which uses constant amounts of two monoclonal antibodies.
Measurement of paraoxonase and arylesterase enzyme activities in serum. PON1 and ARE enzyme activities were measured using commercially available kits(Relassay®, Turkey). The fully automated PON1 activity measurement method consists of two different sequential reagents; the first reagent is an appropriate Tris buffer and it also contains calciumion, which is a cofactor of PON1 enzyme. Phenylacetate was used as a substrate to measure the ARE activity.
Paraoxonase phenotype distribution:
The genetic polymorphism Q/R in codon 192 is responsible for three isotypes: QQ (low activity), QR (intermediate activity), and RR (high activity).The phenotype distribution of PON1 was determined using the dual substrate method. The ratio of paraoxon hydrolysis in the presence of 1 moL/L NaCl (salt stimulated paraoxonase: stPON) to phenylacetate hydrolysis was used to assign individuals to one of the phenotypes. The cut-off values for assigning a participant to a phenotype were <2.0 for QQ, 2.0 to 5.0 for QR and >5.0 for RR [13,14].
Measurement of the total oxidant status of serum
The TOS of the plasma was measured using a novel automated colorimetric measurement method for TOS developed by Erel. The results are expressed in terms of micromolar hydrogen peroxide equivalent per liter (µmol H2O2 Equiv./L) [15].
Measurement of the total antioxidant status of serum
The TAS of the serum was measured using a novel automated colorimetric measurement method for TAS developed by Erel. The results are expressed as micromolar trolox equivalent per liter [15].
Oxidative stress index
The percentage ratio of TOS level to TAS level was accepted as oxidative stress index(OSI) [10]. For calculation, the resulting micromolar unit of TAS was changed to millimoles per liter, and the OSI value was calculated according to the following formula: OSI (arbitrary unit) = TOS(micromolar hydrogen peroxide equivalent per liter)/TAS (micromolar trolox equivalent per liter) [15].
Routine parameters
The levels of triglycerides (TG), total cholesterol (TC), HDL-cholesterol (HDL-C), LDL-cholesterol (LDL-C), uric acid and serum creatinine were determined by using commercially available assay kits(Abbott) with an autoanalyzer (Architect ®c16000, Abbott Diagnostics).
Statistical analysis
Statistical analyses were carried out using the statistical software version 11.5.1.0 (MedCalc, Mariakerke, Belgium). In normal distributed groups the results were presented with a mean and SD. In abnormal distribution groups, the results were presented with medians. The significance of the differences between groups was determined by the Student’s unpaired t-test for normal distributions, and by the Mann–Whitney U-test in abnormal distribution. Pearson correlation coefficient and Spearman correlation coefficient were used to test the strength of any associations between different variables. P values less than 0.05 was accepted as the significance level.
Results
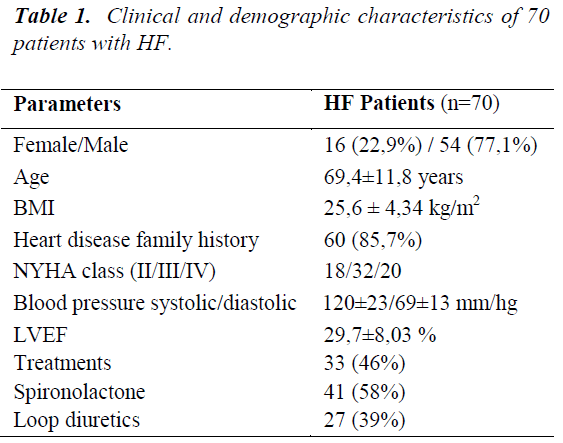
The demographic characteristics, clinical findings, medications and laboratory data of the HF are shown in table 1 and table 2. 70 patients (54 male, 16 female) were enrolled for data collection. The median age was 69.4±11.8 years. 85.7% of patients reported heart disease family history. The mean disease duration of the study group was 10.9 years. Ischemic causes were observed to be prominent in the etiology of the disease (75.7%).
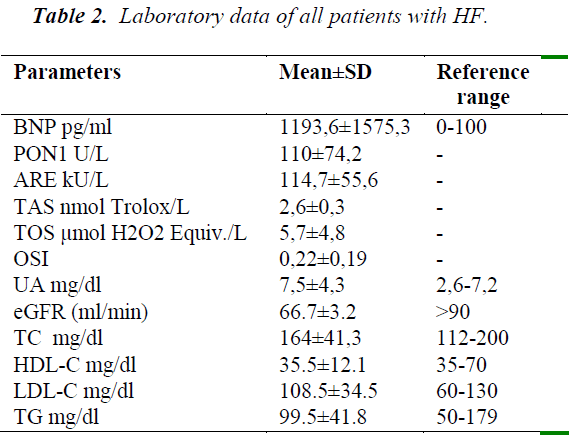
Table 2 includes the overall summary of laboratory data. BNP levels were very high as expected. The uric acid mean was higher than the upper reference range. Serum HDL-C mean was about the lower reference range. Serum creatinine, TC, TG, means were within reference limits. LDL-C mean was above optimal.
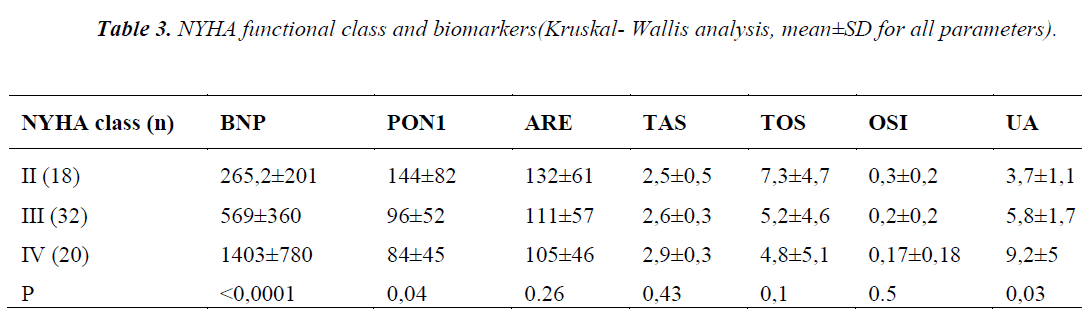
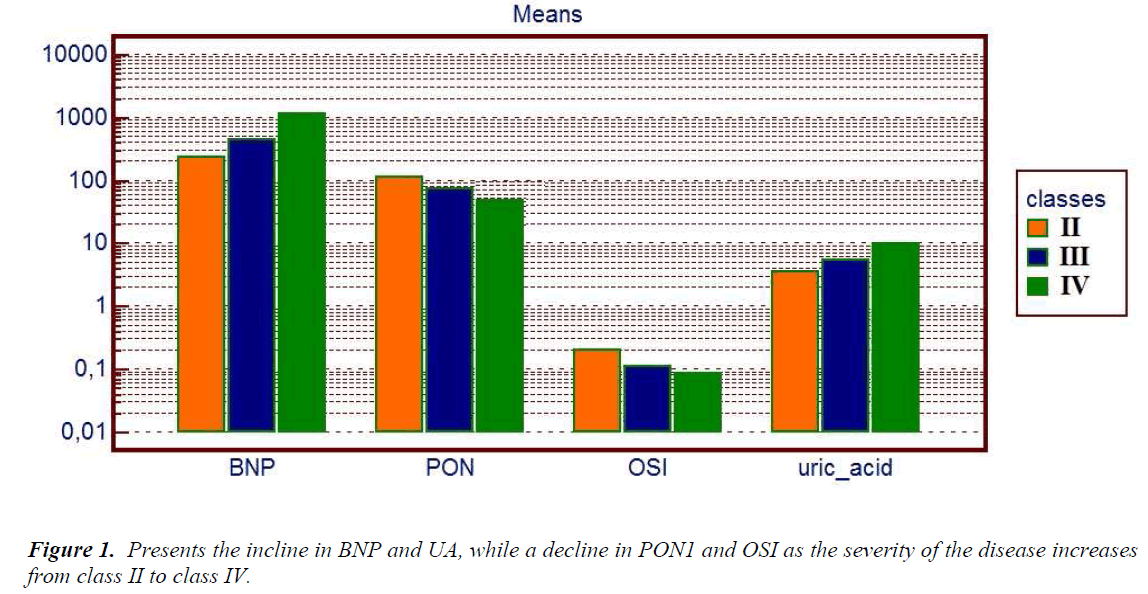
Table 3 shows the laboratory data of patients, divided in 3 groups according to suggestions of NYHA functional class, we found the plasma PON1 activity significantly decreased with the severity of the disease (p=0.04). On the contrary, BNP and UA levels were significantly increased with the severity of the disease (p<0.0001, p=0.03, respectively) (Figure 1). Notably, UA levels in class IV patients were over the upper reference limit, namely a state of hyperuircemia. ARE, TAS, TOS, and OSI did not show any statistically significant difference.
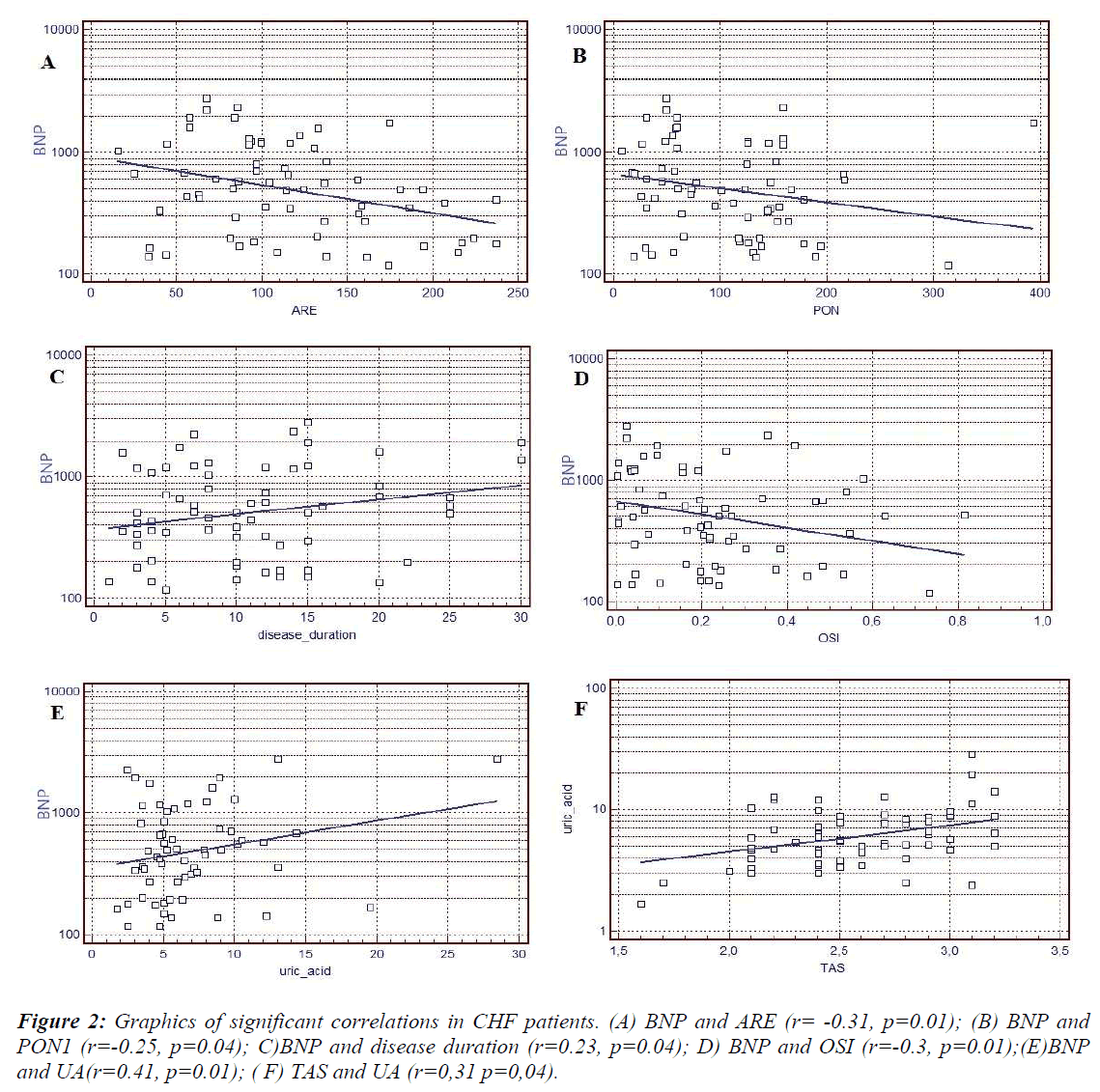
Statistical analysis showed negative correlation between HDL- associated PON1, ARE activities and BNP. Positive correlation between disease duration, UA and BNP was observed. Also, positive correlation was determined between TAS and UA (Figure 2).
The phenotype distribution of PON1 was determined by using the dual substrate method. The resulting ratio was used to assign a phenotype to each participant: QQ, QR and RR. The following phenotype distributions were assessed in the HF patients (n=70): QQ in 36(51,4%) patients, QR in 25(35,7%) patients and RR in 11(12,9%) patients.
However there were no significant differences in between the phenotype distribution and BNP, LVEF(%), HF classes and disease duration.
Statistical analysis showed negative correlation between HDL- associated PON1, ARE activities and BNP. Positive correlation between disease duration, UA and BNP was observed. Also, positive correlation was determined between TAS and UA (Figure 2).
The phenotype distribution of PON1 was determined by using the dual substrate method. The resulting ratio was used to assign a phenotype to each participant: QQ, QR and RR. The following phenotype distributions were assessed in the HF patients (n=70): QQ in 36(51,4%) patients, QR in 25(35,7%) patients and RR in 11(12,9%) patients. However there were no significant differences in between the phenotype distribution and BNP, LVEF(%), HF classes and disease duration.
Discussion
The results of this study suggest that HDL-associated PON1 levels may influence HF. We report for the first time, a notable relationship between HDL-associated antioxidant PON-1 activity and NYHA classification for HF. In the only similar study to ours, Tang et al. reported diminished HDL-associated anti-oxidant PON-1 activity (as monitored by serum ARE activity) in stable patients with impaired LV systolic function [16]. It would appear that, the precise pathophysiologic mechanism of PON1 activity in human chronic HF is still obscure. Our prominent results provide further support for a role for oxidative processes in the disease progression of HF and for a potential anti-oxidant compensatory role of HDL.
The expression and variability of systemic PON1 activities over the natural history of HF is largely unknown, although it is reasonable that reduced serum PON1 activities can adversely lend to disease progression in a various of ways [16,17]. The chiefly descripted mechanism is the association between PON1 activities and protection against lipoprotein oxidation, thereby antagonizing progression of atherosclerotic coronary artery disease, a major cause of heart failure in developed societies [18]. Another potential preventive fitting for HDL-associated PON1 activities may include the possibility for preventing micro-vascular dysfunction as a result of endothelial dysfunction [19]. Phenotypic predisposition has also been suggested as a mechanism for HF development, although few studies have elucidated the genes and genetic variants involved. Moura et.al reported that the PON1 genotype Q192R SNP has an important effect on calcific aortic valve stenosis disease progression [20].
There is little information concerning a direct protective effect of HDL on the heart. Recent mass spectrometric analyses revealed that the protein composition of HDL is complex, thus expanding our understanding of the functions and structures of lipoproteins. HDL is known to remove cholesterol from peripheral tissues in a process known as reverse cholesterol transport [7]. Beyond this function, HDL has been shown to exert cardiovascular protective effects in endothelial and vascular smooth muscle cells by generating a cascade of intracellular signals including the activation of mitogen-activated protein kinase [21]. These properties are mediated by activation of kinases such as protein kinase C(PKC). PKC-dependent phosphorylation modifies Cx43 gap junction channel properties, which are present in ventricular cardiomyocytes, and is involved in cardioprotection [22]. Decreased HDL levels in relation to a higher risk of incident HF has been demonstrated in large epidemiology studies [23]. Raghava S.et.al. reported that elevated non–HDL-C and decreased HDL-C were associated with increased risk of HF [23].
Serum UA levels were frequently observed to be increased in patients with HF and were strongly and independently related to mortality from HF, in previous studies. The possible link between UA overproduction and myocardial damage would appear to be the upregulation of xanthine oxidase (XO) activity. XO and xanthine dehydrogenase are forms of the same enzyme, known as xanthine oxidoreductase. This enzyme is widely distributed in mammalian tissues and with particularly high expression in capillary endothelium. Both forms of xanthine oxidoreductase catalyze the conversion of hypoxanthine to xanthine and xanthine to uric acid, however only the oxidase form generates O2•- and H2O2. XO may also contribute to oxidative damage in myocardium [24,25].
Hyperuricaemia may also promote oxygenation of lowdensity lipoprotein cholesterol and may facilitate lipid peroxidation, increasing the production of oxygen free radicals [26,27]. In this study, the class IV HF patients did have hiperuricemia even though they had normal renal function and no hiperlipidemia. In the current study, the NYHA class IV HF patients also showed a 2.48-fold higher serum levels of uric acid than the HF patients class II, as shown in Table 3. Serum uric acid would have a protective antioxidant activity. This action could help to reduce or counteract the processes that cause or appear as a result of HF [26-28]. However, these protective properties would disappear in the intracellular environment or in highly hydrophobic areas such as atherosclerotic plaques and adipose tissue. In a previous study by Leyva F et al. , serum UA levels were increased in parallel to disease severity in HF patients, and were inversely associated with NYHA disease categories [29].
A variety of enzymatic and non-enzymatic processes can generate ROS in mammalian cells. Some of the most important sources are the mitochondrial respiratory chain, NADP oxidases, XO, lipoxygenase, uncoupled nitrous oxide synthase and myeloperoxidase. Considerable data indicate that ROS and oxidative stress are important features of cardiovascular diseases including atherosclerosis, hypertension, and HF [27,30]. As can be seen best, oxidative stress results from an imbalance between ROS generation and antioxidant defense mechanisms. Therefore, impaired antioxidant defense mechanisms (i.e PON1 and ARE) or increased concentrations of some endogenous antioxidants (i.e UA and TAS) may generate balance ROS levels in patients with HF.
Although this study was carried out with a small number of participants, this is the first report to present a relation of cardiac biomarker BNP and redox potential in individual HDL –associated PON1 levels in HF. We further demonstrated a correlation between low serum PON1, ARE activities and BNP levels in these patients. Surprisingly, in this study, class IV HF patients showed higher TAS levels, decreased PON1, ARE activities and increased BNP, higher UA levels than lower class (II and III) HF patients. Also negative correlations between some biomarkers (PON1, TOS and OSI) and NYHA categories were observed. Unfortunately, very few studies on this issue are available to predict strict comparisons [31,32]. Although they did not show any correlations, antioxidative parameters (TAS and UA), and their alterations in antioxidative levels and disease duration were well correlated [33,34].
Serum BNP levels were increased in the NYHA class IV patients vs. class II patients, in line with previous studies (p<0.0001) [35-37]. Serum levels of PON1 were lower in class IV patients than in class II patients (p=0.04). These results suggest enhanced oxidative stress during worsening of HF. We found, however, significant correlations between the NYHA classes (II-IV) and PON1 activity, TOS and OSI levels. These analyses may suggest that oxidative stress is enhanced even with mild NYHA functional classes.
Further investigations into the relationship of serum ARE activity ,BNP and disease duration are warranted, as are studies aimed at modulating PON-1 activity as a means of potentially protecting the failing heart from disease progression. Finally we would propose the hypothesis that NYHA classes of HF promotes free radical generation and UA synthesis, presumably by the failing myocardium.
References
- Velagaleti RS, Massaro J, Vasan RS, Robins SJ,Kannel WB, Levy D. Relations of lipid concentrations to heart failure incidence: the Framingham Heart Study. Circulation. 2009 Dec 8; 120(23): 2345-2351.
- Bennett DA, EliaszTK, Forbes A, Kiszely A, KhoslaR, Petrinic T, Praveen D, Shrivastava R, Xin D, Patel A, Macmahon S, Rahimi K. Study protocol: systematic review of the burden of heart failure in low- and middle-income countries. Syst Rev. 2012; 1(1): 59.
- Rogowski O, Shnizer S, Wolff R, Lewis BS, Amir O. Increased serum levels of oxidative stress are associated with hospital readmissions due to acute heart failure. Cardiology. 2011; 118(1): 33-37. doi: 10.1159/000324192. Epub 2011 Mar 11.
- Kumar EP, Mukherjee R, Senthil R, Parasuraman S, Suresh B. Evaluation of oxidative stress and antioxidant status in patients with cardiovascular disease in rural populations of the nilgiris, South India. ISRN Pharmacol. 2012; 2012: 941068
- Ellidag HY, ErenE, Yilmaz N, Cekin Y. Oxidative stress and ischemia-modified albumin in chronic ischemic heart failure. Redox Rep. 2014; 19(3): 118-123.
- Lakshmi SV, Padmaja G, Kuppusamy P, Kutala VK. Oxidative stress in cardiovascular disease.Indian J BiochemBiophys. 2009; 46(6): 421-440.
- Eren E, Yilmaz N, Aydin O. High Density Lipoprotein and it’s Dysfunction. Open Biochem J. 2012; 6: 78-93.
- Yilmaz N. Relationship between paraoxonase and homocysteine: crossroads of oxidative diseases. Arch Med Sci. 2012; 8(1): 138-153.
- Yilmaz N, Aydin O, Yegin A, Tiltak A, Eren E. Increased levels of total oxidant status and decreased activity of arylesterase in migraineurs. ClinBiochem. 2011; 44(10-11): 832-837.
- Yilmaz N, Aydin O, Yegin A, Tiltak A, Eren E, AykalG. Impaired oxidative balance and association of blood glucose, insulin and HOMA-IR index in migraine. Biochem Med (Zagreb). 2011; 21(2): 145-151.
- Palazzuoli A, Gallotta M, Quatrini I, Nuti R. Natriuretic peptides (BNP and NT-proBNP): measurement and relevance in heart failure. VascHealth Risk Manag.2010; 6: 411-418.
- American Heart Association (1994) AHA medical/scientific statement. 1994 revisions to classification of functional capacity and objective assessment of patients with diseases of the heart.Circulation 90(1): 644-645.
- Patel AB, Pal R, Dewan A. Distribution profile ofparaoxonase phenotypes among the Gujaratis. Indian J Hum Genet. 2007; 13(2): 65-68.
- Eckerson HW, Wyte CM, La Du BN. The human serum paraoxonase/arylesterase polymorphism.Am J Hum Genet. 1983; 35(6): 1126-1138.
- Erel O.A new automated colorimetric method for measuring total oxidant status. ClinBiochem. 2005; 38(12): 1103-1111.
- Tang WH, Wu Y, Mann S, Pepoy M, Shrestha K, Borowski AG, Hazen SL. Diminished antioxidant activity of high-density lipoprotein-associated proteins in systolic heart failure. Circ Heart Fail. 2011; 4(1): 59-64.
- Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, Fu X, Shao M, Brennan DM, Ellis SG, Brennan ML,Allayee H, Lusis AJ, Hazen SL. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 2008; 299: 1265-1276
- Mehra MR, Uber PA, Lavie CJ, Milani RV, Park MH, Ventura HO. High-density lipoprotein cholesterol levels and prognosis in advanced heart failure. J Heart Lung Transplant 2009; 28: 876–880.
- Gur M, Yildiz A,Demirbag R, Yilmaz R, Aslan M, Ozdogru I, ErelO. Paraoxonase and arylesteraseactivities in patients with cardiac syndrome X, and their relationship with oxidative stress markers. CoronArtery Dis 2007; 18: 89-95.
- Moura LM, Faria S, Brito M, Pinto FJ, Kristensen SD, Barros IM, Rajamannan N, Rocha-Gonçalves F. Relationship of PON1 192 and 55 gene polymorphisms to calcific valvular aortic stenosis. Am J CardiovascDis. 2012; 2(2): 123-132.
- Frias MA, James RW, Gerber-Wicht C, Lang U. Native and reconstituted HDL activate Stat3 in ventricular cardiomyocytes via ERK1/2: role of sphingosine-1- phosphate. Cardiovasc Res. 2009 May 1; 82(2): 313- 323.
- Morel S, FriasMA, Rosker C, James RW, Rohr S, Kwak BR. The natural cardioprotective particle HDL modulates connexin43 gap junction channels. Cardiovasc Res. 2012; 93(1): 41-49.
- Velagaleti RS, Massaro J, Vasan RS, Robins SJ, Kannel WB, Levy D. Relations of lipid concentrations to heart failure incidence: the Framingham Heart Study. Circulation 2009; 120(23): 2345-2351.
- Ioachimescu AG, Brennan DM, Hoar BM, Hazen SL,Hoogwerf BJ. Serum uric acid is an independent predictor of all-cause mortality in patients at high risk of cardiovascular disease: a preventive cardiology information system (PreCIS) database cohort study. Arthritis Rheum. 2008; 58(2): 623-630.
- Hamaguchi S, Furumoto T, Tsuchihashi-Makaya M, Goto K, Goto D, Yokota T, Kinugawa S, Yokoshiki H, Takeshita A, Tsutsui H; JCARE-CARD. Investigators.Hyperuricemia predicts adverse outcomes in patients with heart failure. Int J Cardiol. 2011; 151(2): 143-147.
- Alimonda AL, Núñez J, Núñez E, Husser O, Sanchis J, Bodí V, Miñana G, Robles R, Mainar L, Merlos P, Darmofal H, LlácerA. Hyperuricemia in acute heart failure. More than a simple spectator?Eur J Intern Med. 2009; 20(1): 74-79
- Sugamura K, Keaney JF Jr. Reactive oxygen species in cardiovascular disease. Free RadicBiol Med. 2011; 51(5): 978-992.
- Zhao QY, Yu SB, Huang H, Cui HY, Qin M, Huang T, Huang CX. Serum uric acid levels correlate with atrial fibrillation in patients with chronic systolic heart failure. Chin Med J (Engl). 2012 May; 125(10): 1708- 1712.
- Leyva F, Anker S, Swan JW, Godsland IF, WingroveCS, Chua TP, Stevenson JC, Coats AJ. Serum uric acid as an index of impaired oxidative metabolism in chronic heart failure.Eur Heart J. 1997 May; 18(5): 858-865.
- Landmesser U, Spiekermann S, Dikalov S, Tatge H, Wilke R, Kohler C, Harrison DG, Hornig B, Drexler H. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthineoxidaseand extracellular superoxide dismutase. Circulation. 2002; 106(24): 3073-3078.
- TsarouhasK, Tsitsimpikou C, Haliassos A, Georgoulias P, Koutsioras I, Kouretas D, Kogias J, Liosis I, Rentoukas E,Kyriakides Z. Study of insulin resistance, TNF-a, total antioxidant capacity and lipid profile in patients with chronic heart failure under exercise. In Vivo. 2011; 25(6): 1031-1035.
- Greig D, Alcaino H, Castro PF, Garcia L, Verdejo HE, Navarro M, López R, Mellado R, Tapia F, GabrielliLA, Nogerol C, Chiong M, Godoy I, Lavandero S. Xanthine-oxidase inhibitors and statins in chronic heart failure: effects on vascular and functional parameters. J Heart Lung Transplant. 2011; 30(4): 408-413.
- Bishu K, Deswal A, Chen HH, LeWinter MM, LewisGD, Semigran MJ, Borlaug BA, McNulty S, Hernandez AF, Braunwald E, Redfield MM. Biomarkers in acutely decompensated heart failure with preserved or reduced ejection fraction. Am Heart J. 2012; 164(5): 763-770.
- Harzand A, Tamariz L, Hare JM. Uric acid, heart failure survival, and the impact of xanthine oxidase inhibition. Congest Heart Fail. 2012 May-Jun; 18(3): 179-182.
- Gaggin HK, Januzzi JL Jr. Biomarkers and diagnostics in heart failure. BiochimBiophysActa.2013 Jan 9.
- Palazzuoli A, Gallotta M, Quatrini I, Nuti R. Natriuretic peptides (BNP and NT-proBNP): measurement and relevance in heart failure. VascHealth Risk Manag.2010; 6: 411-418.
- Elasfar A. Correlation between plasma N-terminal probrainnatriuretic peptide levels and changes in New York Heart Association functional class, left atrial size, left ventricular size and function after mitral and/or aortic valve replacement. Ann Saudi Med. 2012; 32(5): 469-472.
- Nagayoshi Y, Kawano H, Hokamaki J, Uemura T, Soejima H, KaikitaK, Sugiyama S, Yamabe H, ShiojiI, Sasaki S, Kuroda Y, Ogawa H. Differences in oxidative stress markers based on the aetiology of heart failure: comparison of oxidative stress in patients with and without coronary artery disease. Free Radic Res. 2009; 43(12): 1159-1166.
- Radovanovic S, Krotin M, Simic DV, Mimic-Oka J, Savic-RadojevicA, Pljesa-Ercegovac M, Matic M, Ninkovic N, Ivanovic B, Simic T. Markers of oxidative damage in chronic heart failure: role in disease progression. Redox Rep. 2008; 13(3): 109-116.
- Freudenberger RS, Schwarz RP Jr, Brown J, Moore A, Mann D, Givertz MM, Colucci WS, Hare JM. Rationale, design and organisation of an efficacy and safety study of oxypurinol added to standard therapy in patients with NYHA class III - IV congestive heart failure. Expert OpinInvestig Drugs. 2004; 13(11): 1509-1516.