Research Article - Journal of Biochemistry and Biotechnology (2021) Volume 4, Issue 4
The rejuvenating effects of Astragalus membranaceus and Centella asiatica saponins on human skin.
Wen-Liang Chang1*, Su-Fen Huang2, Ching-Hui Oh3, Tsu-Chung Chang2*
1Departmen of Pharmacy, National Defense Medical Center, Taipei, Taiwan
2Department of Biochemistry, National Defense Medical Center, Taipei, Taiwan
3Department of Anesthesiology, Cheng-Hsin General Hospital, Taipei, Taiwan
- Corresponding Author:
- Wen-Liang Chang
Department of Pharmacy
National Defense Medical Center
Taipei, Taiwan
E-mail: wlchang@mail.ndmctsgh.edu.tw
Tsu-Chung Chang
Department of Biochemistry
National Defense Medical Center
Taipei, Taiwan
E-mail: tcchang@ndmctsgh.edu.tw
Accepted date: July 27, 2021
Citation: Chang WL, Huang SF, Oh CH, et al. The rejuvenating effects of Astragalus membranaceus and Centella asiatica saponins on human skin. 2021;4(4):1-6.
Abstract
Collagen and hyaluronic acid presented in skin cells play a critical role in how skin looks. Only a small portion of collagen and hyaluronic acid contained in skin products can be absorbed and utilized by skin cells. Topical skin care products containing collagen does not significantly improve the skin texture, as the collagen molecules cannot penetrate the epidermis, due to their high molecular weight. Topical skin care products with Astragalus membranaceus and Centella asiatica display a wide range of beneficial effects, such as improved wound healing, immunomodulation, and enhanced collagen stimulation. To evaluate the rejuvenating effects of a proprietary formulation of Astragalus membranaceus and Centella asitica Saponins (ACS) on human skin, in vitro and human experiments were performed. In vitro studies indicated that ACS increased the collagen and hyaluronic acid synthesis and secretion and decreased the MMP-1 synthesis induced by UVB in hacat and HDF cells. In addition, ACS enhanced the skin elasticity by increasing the proline and glucosamine absorption and significantly increased the telomerase activity. Moreover, ACS reduced wrinkles and melanin in human subjects. In conclusion, the in vitro and human studies demonstrated that ACS has a rejuvenating effect on human skin due to its efficacy in enhancing the skin cells’ ability to synthesize greater amounts of collagen and hyaluronic acid, as well as increasing the uptake of proline and glucosamine in hacat and HDF cells.
Keywords
Saponins, Dermal fibroblasts, Hydrating ingredient, Rejuvenating effects, UV protection
Introduction
Skin rejuvenation generally refers to the reversal of aging of human facial dermal areas, namely repair of the damage that is associated with aging or replacement of damaged facial tissue with new tissue. The molecular mechanism of skin aging maybe associated with free radical-induced damage and over-expression of pro-inflammatory molecules, leading to a state of chronic inflammation [1]. Various skin rejuvenation techniques have been used with the aim of restoring a youthful appearance to the face or other dermal areas. On the face, localized facial concerns such as wrinkles, pigmentation defects, scars, and looseness are often the target of treatment. Products marketed to reduce or prevent signs of skin aging are intended for topical application due to less invasiveness and ease of use. Recently, the increased use of natural herbal ingredients accelerated the developments of new skin care products [2-4]. Accordingly, there is a rising demand for effective and natural skin rejuvenating compositions.
Astragalus membranaceus and Centella asiatica are popular medicinal herbs for improving various skin conditions in traditional Chinese medicine [5,6]. They have been included in topical skin care products even though their mechanisms of actions are not well-defined. The main constituents of these two herbs are the cycloartane- type Saponins [7,8]. Astragalus or astragalosides have been used as cardioprotective, immune-modulatory, and wound-healing agents [8,9]. A number of clinical and physiological effects of Astragalus have been investigated.
The primary active constituents of Centella Asiatica are saponins, which include asiaticosides, in which a trisaccharide moiety is linked to the aglycone asiatic acid, madecassoside and asiatic acid [10]. These triterpene saponins and their sapogenins are responsible for the wound healing and vascular effects of Centella asiatica by inhibiting the production of collagen at the wound site [11]. They have been reported to possess wound healing activity by increasing collagen formation and angiogenesis [12]. Apart from showing their ability to increase collagen synthesis in different cell types, the asiaticosides were shown to increase the tensile strength of the newly formed skin, furthering the healing of wounds [13]. Overall, Astragalus membranaceus and Centella Asiatica are generally considered as safe. Given the potential beneficial activity of Astragalus membranaceus and Centella Asiatica for skin health, the rejuvenating effects of a specific formulation (ACS) consisting of the enriched fractions of Astragalus membranaceus and Centella Asiatica was investigated. A series of in vitro studies were performed using spontaneously transformed human keratinocyte (HaCaT) and Human Dermal Fibroblasts (HDF) from healthy human subjects. The knowledge gained from these studies will provide valuable information for the development of natural and effective topical rejuvenating agents.
Materials and Methods
ACS is a mixture of dried extracts of Astragalus membranaceus and Centella Asiatica roots; its production is compliant with current Good Manufacturing Practice. The final blend is Off-white to beige powder and is standardized to contain ≥ 0.25% of total saponins. The ACS used in the studies was provided by NuLiv Science USA, Inc. (Brea, CA, USA). The cream that contains 5% of ACS formulation was used in this study.
HaCaT and HDF cell culture
Spontaneously transformed human keratinocyte HaCaT cell line was obtained from American Type Culture Collection. HaCaT cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 4.5 g/L glucose, 0.584 g/L glutamine, 10% fetal bovine serum, 3.7% sodium bicarbonate, 100 IU/mL penicillin, 100 g/mL streptomycin, and 1% nonessential amino acids. Primary HDF were cultured in medium 106 (Cascade Biologics, Portland, USA). The medium 106 was supplemented with 10% fetal bovine serum, 100 IU/mL penicillin, 100 g/mL streptomycin. The cells from passages 4 to 10 were used for this study. Both cells grew at 37ºC in a humidified incubator under 5% CO2 and 95% air in culture, and culture medium was replaced every other day. After confluence, the cells were sub-cultured following trypsinization.
Endogenesis collagen studies
Collagen I and MMPs secretion assay: The cells were plated on 6 cm dishes at a density of 0.3 × 106 cells/ dish for 24 h prior to any treatment. The cells were then treated with ACS at indicated dosages for another 48 h. The conditioned culture mediums were then centrifuged, 2-fold concentrated and collected for assay of collagen I and MMPs secreted into the culture medium [14]. Western blot analysis was carried out on both the cultured medium and the cell lysates as follows: The cells were washed and lysed in lysis buffer. Protein concentration of the samples was measured using the Bicin-Choninic Acid (BCA) protein assay kit according to the manufacturer’s protocol (Pierce, Rockford, IL, USA). Protein samples of concentrated conditioned medium or cell lysate supernatants were mixed with an appropriate volume of SDS sampling buffer and separated by SDS-PAGE gel. The protein bands separated in the SDS-PAGE gel were blotted onto a polyvinylidene fluoride membrane. The blotted PVDF membrane was washed twice and blocked in freshly prepared Tris-Buffered Saline (TBS) containing Tween-20 and 7% skim milk at room temperature. The PVDF membrane was then incubated with either antibodies against collagen I or the housekeeping protein α-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA). Horseradish peroxidase-conjugated anti-goat antibody was used as secondary antibody. Signals were visualized by an enhanced chemiluminescence kit (Clonetech, Palo Alto, CA, USA) followed by exposure to X-ray films.
Proline uptake assay: HaCaT cells were seeded into a 24- well plate and cultured for 24 h. The cells were then treated in the absence (solvent control) or presence of the ACS at various doses for another 48 h. The treated cells were then washed once with PBS and incubated in Amino Acid Free Medium (AAFM) for another 30 min. The treated cells were then replaced with fresh AAFM containing of ascorbate and total of L-proline containing [3H] Proline (American Radiolabelled Chemicals Inc, ARC, St. Louis, MO, USA) [15]. At designated time intervals, the cells were washed with AAFM containing cold proline and then lysed in SDS solution. Cell lysates were centrifuged. Intracellular proline uptaken by the cells was determined by transferring the cell lysate to filter-bottomed UniFilter plates (Perkim-Elmer) and counting. The samples were measured for their protein concentrations using the BCA protein assay kit as described above. The amount of proline accumulated in the cells was calculated and normalized to protein concentration. And cellular uptake rate was expressed as nmole of L-proline per minute per milligram of cell protein (nmole/min/μg).
UV irradiation assay: To characterize the effects of ACS on the expression level of MMPs in HDF cells were first treated with or without ACS at various doses for 24 h. The cells were washed with phosphate-buffered saline (PBS) and irradiated with 10-100 mJ/cm2 of UV-B (312 nm) for HDF and HaCaT cells, respectively, using the UV light irradiator (UVI tec unlimited, Cambridge, England). The cells were then washed and incubated with serum free medium for 24 h before harvested for analysis. The proteolytic activities of MMPs from HDF cells were measured essentially as described elsewhere [16]. The samples were measured for their protein concentration using the bicinchoninic acid (BCA) protein assay kit as described. Samples of concentrated conditioned medium and cell lysates were mixed with non-reducing electrophoresis loading buffer and subjected to electrophoresis under non-reducing conditions.
Endogenesis Hyaluronic Acid (HA) studies
Hyaluronic acid measurement: HaCaT cells were seeded and incubated in 24-well plates to confluency. Immediately before experiments, cells were washed twice with serum- free medium to completely remove HA accumulated during cell growth. The cells were then cultured with or without different compounds in 0.5 ml serum-free medium for 48 h. At the indicated time, aliquots of medium were removed, centrifuged, and supernatants were analyzed for HA using an Enzyme-Linked Immuno Sorbent Assay (ELISA) kit (Echelon Bioscience, Salt Lake, UT). Relative levels of transcripts expression in HaCaT cells were determined by real-time quantitative reverse transcriptase polymerase chain reaction (qRT-PCR). qRT-PCR was performed using the Applied Biosystem 7300 system and pre-developed TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA, USA). The reaction mixture contained of serially diluted cDNA, TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA, USA), and either human HAS-2, MMP-1 or GAPDH primer mix (Applied Biosystems, Foster City, CA, USA). Two independent triplicate experiments were performed for the selected genes. Expression of HAS-2 was normalized to GAPDH expression in each sample [17,18].
Glucosamine uptake assay: Glucosamine is the primary building block of HA. Therefore, a measurement of glucosamine uptake by the cells suggests synthesis of HA in human cells. In glucosamine uptake test, HaCaT cells were seeded into 24-well plate and cultured for 24 h. The cells were then treated in the absence (solvent control) or presence of ACS at various doses for another 48 h. The treated cells were then washed twice with PBS and incubated in Glucose and Serum Free Medium (GSFM). After 2 h, the cells were replaced with fresh GSFM containing 0.2 μCi of [14 C]-Glucosamine (American Radio labelled Chemicals Inc, ARC, St. Louis, MO, USA). At the designated time interval, the cells were washed twice with GSFM containing cold glucosamine and then lysed in SDS solution. The samples were measured for their protein concentration using the BCA protein assay kit as described above. The amount of glucosamine accumulated in the cells was calculated and normalized to protein concentration and uptake rate was expressed as counts per minute per microgram of cell protein (cpm/ min/μg) [19].
Telomerase activity assay
In telomerase activity test, HaCaT cells were seeded into 6 well plates for 24 hours. The cells were then treated in the absence (solvent control) or presence of ACS for another 24 h. Telomerase of the samples was measured by TRAPEZE RT Telomerase Detection Kit according to the manufacturer’s protocol (Merck Millipore, USA). The reactions were amplified according to indicated condition. Three independent triplicate experiments were performed for telomerase assay [20].
Human studies
Test subjects: The human trial complies with the guidelines for human studies and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. All subjects have given their written informed consent and that the study protocol was approved by the institute’s committee on human research. Nineteen healthy volunteers, aged 38 years and older, completed the 4 weeks study. The test subjects were asked not to put any other cosmetic products on their faces during the whole period of the study. All the tests were conducted at a room temperature ranged from about 24ºC to 28ºC, and at a relative humidity ranged from about 50% to 60%. Each measurement was taken from a test spot having a diameter of about 2 cm at three selected areas (forehead, corner of the eye and upper cheek). The physical properties (skin melanin content and skin roughness) of the skin were evaluated 30 minutes after cleansing the subjects’ faces to provide the measurements [21]. A suitable amount (e.g. size of a peanut) of an ACS containing cream was applied evenly on subject’s face in the morning and evening for 4 weeks.
Skin melanin measurements: The Mexameter® MX18 spectrophotometer (Courage+Khazaka, 91 Mathias- Bruggen-Str., Cologne, Germany) emits light of three defined wave lengths (568 nm, 660 nm and 870 nm) and a receiver measures the light reflected by the skin. The results correspond to the spectral absorption peaks of melanin.
Skin roughness (wrinkle) measurement: The skin roughness was measured using a Skin Visio meter SV (Courage+Khazaka electronic GmbH, Koln, Germany) based on light transmission through a thin replica comprising of blue-dyed two-component silicone of which the light absorption was known. With the thin replica inserted in a modified slide projector, light from a neon lamp penetrated the replica and was absorbed depending on the thickness of the silicone material. A video sensor charge couple device and a black and white (b/w) CMOS- camera with a resolution of 640 × 480 pixels measured the amount of locally transmitted light, and the light intensity was calculated according to Lambert and Beer’s law of absorption.
Calculations and statistical analysis: All data are presented as the mean ± SEM. Significant differences between group’s means were determined by one-way ANOVA. Statistical comparisons were made by paired t-test for paired data. Statistical significance was accepted at a 0.05 level of probability.
Results
Endogenesis collagen studies
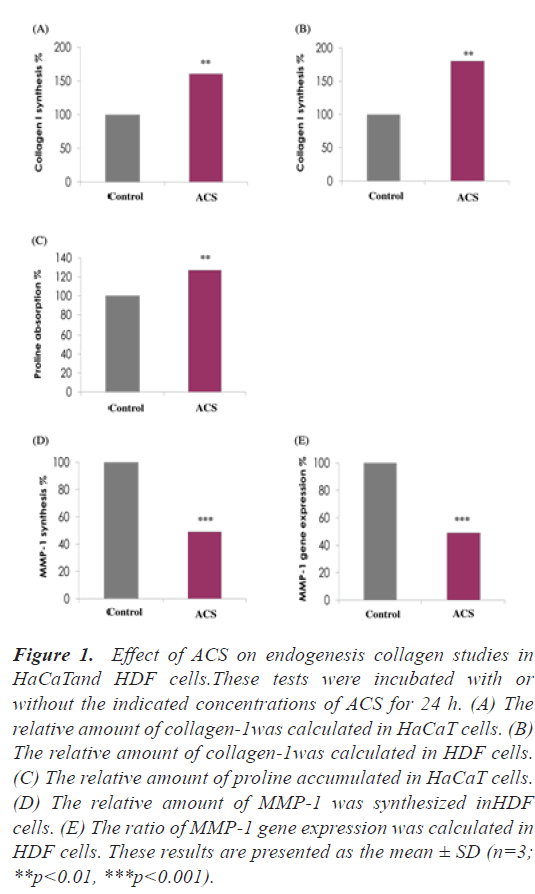
ACS enhances collagen I synthesis in epidermal and dermal cells: To assess the effects of ACS on collagen-I synthesis, HaCaT and HDF cells were treated with a placebo or ACS. The results indicated that ACS increased the collagen I synthesis in epidermal and dermal cells. Twenty-four hours after incubation with ACS, collagen-I synthesis increased by 60% in HaCaT cells and 80% in HDF cells, respectively (relative to control cells, p<0.01) (Figure 1A and 1B).
Figure 1: Effect of ACS on endogenesis collagen studies in HaCaTand HDF cells.These tests were incubated with or without the indicated concentrations of ACS for 24 h. (A) The relative amount of collagen-1was calculated in HaCaT cells. (B) The relative amount of collagen-1was calculated in HDF cells. (C) The relative amount of proline accumulated in HaCaT cells. (D) The relative amount of MMP-1 was synthesized inHDF cells. (E) The ratio of MMP-1 gene expression was calculated in HDF cells. These results are presented as the mean ± SD (n=3; **p<0.01, ***p<0.001).
ACS increase proline uptake in epidermal cells: To evaluate whether the building block of collagen is increased in epidermal (HaCaT) cells, the effect of ACS on proline absorption was studied. We found that ACS significantly enhanced proline absorption by 27% compared to the control cells (Figure 1C) (p<0.01).
ACS inhibited MMP-1 synthesis and gene expression in dermal (HDF) cells under UV irradiation: To assess the effects of ACS on MMP-1 synthesis and gene expression, HDF cells were treated with either placebo or ACS. The results indicated that ACS decreased MMP-1 synthesis and gene expression in dermal cells. Twenty-four hours after incubation with ACS, MMP-1 synthesis decreased by 50% and MMP-1 gene expression decreased by 51% in HDF cells (relative to control cells, p<0.001) (Figure 1D and 1E).
Endogenesis hyaluronic acid studies
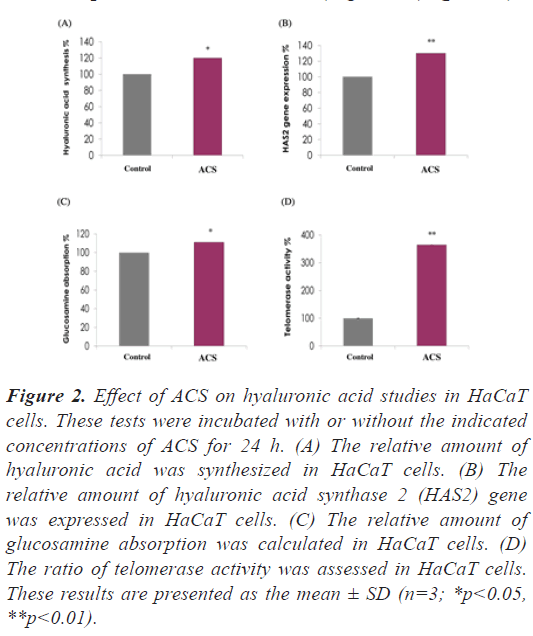
ACS significant increased hyaluronic acid synthesis and glucosamine uptake in epidermal cells: In addition to its effect on collagen augmentation, the effects of ACS on hyaluronic acid level as well as glucosamine which act as the building block of hyaluronic acid was studied. The results shown in Figure 2A and 2B indicated a significant increase of hyaluronic acid synthesis and hyaluronic acid synthase 2 (HAS2) gene expression in epidermal (HaCaT) cells by 20% and 30% (p<0.05, p<0.01, respectively) compared to the control cells. In addition, ACS increased glucosamine absorption in epidermal (HaCaT) cells by 11% compared to the control cells (Figure 2C), (p<0.05).
Figure 2: Effect of ACS on hyaluronic acid studies in HaCaT cells. These tests were incubated with or without the indicated concentrations of ACS for 24 h. (A) The relative amount of hyaluronic acid was synthesized in HaCaT cells. (B) The relative amount of hyaluronic acid synthase 2 (HAS2) gene was expressed in HaCaT cells. (C) The relative amount of glucosamine absorption was calculated in HaCaT cells. (D) The ratio of telomerase activity was assessed in HaCaT cells. These results are presented as the mean ± SD (n=3; *p<0.05, **p<0.01).
ACS significant increased telomerase activity in epidermal cells: Increasing evidence indicates that telomerase plays a significant role in the maintenance of skin function and proliferation, thus the effects of ACS on telomerase activity in HaCaT cells was evaluated. ACS significantly increased the telomerase activity in epidermal cells by 265% compared to the control cells (Figure 2D), (p<0.01).
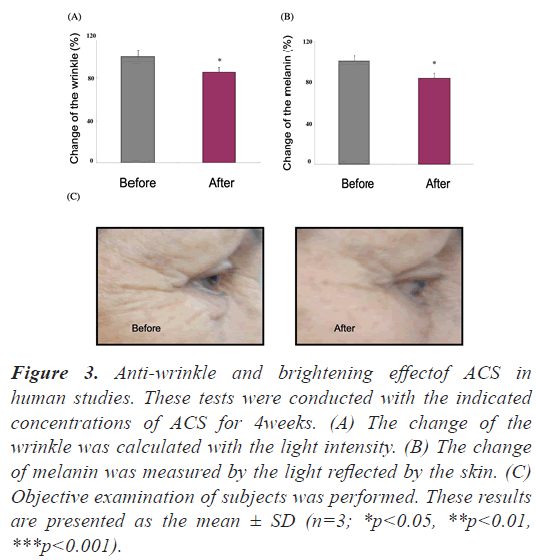
In human studies, ACS has demonstrated to reduce wrinkle and melanin volume compared to pre- treatment baseline: To further examine the effects of ACS on human facial skin, nineteen healthy volunteers, aged 38 years and older, participated in a 4 week study. Tests including skin melanin content and roughness were made based on reflection/absorption of light using specific instruments. In this human study, ACS reduced wrinkle volume of the test subjects by 14.8% and melanin by 16.6% (Figure 3), (p<0.05).
Figure 3: Anti-wrinkle and brightening effectof ACS in human studies. These tests were conducted with the indicated concentrations of ACS for 4weeks. (A) The change of the wrinkle was calculated with the light intensity. (B) The change of melanin was measured by the light reflected by the skin. (C) Objective examination of subjects was performed. These results are presented as the mean ± SD (n=3; *p<0.05, **p<0.01, ***p<0.001).
Discussion
Collagen is the major building block of human dermis, with compressibility characteristics, giving the skin a tensile, hydrated appearance. Specifically, type I collagen are found in abundance, composing almost 90% of the total collagen in the skin [22]. Apparently, the abundance of collagen 1 may improve the associated dermal laxity characteristic of aging skin. Ultraviolet (UV) B irradiation induces the production of matrix metalloproteinases (MMPs) which are responsible for the degradation of extracellular matrix proteins [23]. MMP-1, a collagenase, plays crucial roles in skin inflammation and wound healing. Over-expression of MMP-1 results in the delay of re-epithelialization in dermal cells as there are high levels of MMP-1 in chronic non-healing wounds [24]. Inhibition of MMP-1 production and gene expression may prove effective in the treatment of photo-aged skin. Non-essential amino acids such as proline and hydroxyproline are vital for collagen biosynthesis, structure, and strength. Their cyclic structure constrains the rotation of the polypeptide collagen chain, creating and strengthening the helical characteristic of the molecule [25]. In our in vitro studies, ACS was shown to possess positive effects for increasing the collagen 1 and proline production, lowering the MMP-1 production and gene expression.
Hyaluronic Acid (HA) appears in the periphery and at interfaces of collagen and elastin fibers where it facilitates holding collagen and extracellular matrices in proper configuration. In aged skin, these connections with hyaluronic acid are particularly weakened, contributing to the disorganization of collagen and elastin fibers [26]. HA is naturally synthesized by a class of hyaluronan synthases, which classify into three types: HAS1, HAS2, and HAS3 [27]. HAS2 is able to synthesize high molecular weight hyaluronic acid, which is thought to have an anti-inflammatory effect. In addition, glucosamine plays a key role in structure and function as well as being a precursor substrate for the biosynthesis of hyaluronic acid. Enhanced glucosamine production has been shown to accelerate wound healing, improve skin hydration, and decrease wrinkles [28]. Therefore, the increase of hyaluronic acid, HAS2 gene expression and glucosamine, by ACS has demonstrated promising effects for rejuvenating aged skin. Telomere length, a marker of cellular aging that decreases with age, has been associated with aging-related diseases. Telomerase activation has been suggested to be an anti- aging modulator that can play a role in the treatment of aging-related diseases. Our finding is in accordance with the fact that Centella asiatica extract significantly increased telomerase activity in HeLa cells as shown by Tsoukalas et al. suggesting that Centella asiatica extract may improve telomere maintenance [29]. Furthermore, the human trial showed that ACS reduces the wrinkle by 14.8% and melanin by 16.6% in human subjects compared to pre-treatment baseline. Aging is a natural progressive physiological change that results in declines in biological function and the ability to adapt to metabolic stressors. The reduction of skin elasticity reduces skin thickness with concomitant decreases in hyaluronic acid and collagen beneath the skin. The reduction of wrinkle formation is likely due to the increased collagen and hyaluronic acid synthesis and secretion demonstrated in the in vitro studies. Moreover, our study also showed that ACS effectively decreased the formation of melanin, indicating the promising photoprotective effect.
Conclusion
In conclusion, ACS that contains a standardized blend of natural saponin compounds from Astragalus membranaceus and Centella asiatica may have skin rejuvenating effects due to its ability to increase the endogenous collagen and hyaluronic acid synthesis and secretion, proline and glucosamine uptake, telomerase activation, and MMP inhibition.
Acknowledgements
This work was supported by grants from the Cheng- Hsin General Hospital (CH-NDMC-106-15 and CH- NDMC-107-2 to T-C.C.) and the Ministry of National Defense (MAB-108-056, MAB-109-055, MND-MAB-110-118 to T-C.C.), Taipei, Taiwan, ROC. We are grateful to NuLiv Science USA, Inc. for generous assistance in this work.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Author Contributions
S-F.H., C-H.O., W-L.C., and T-C.C. participated in the concept of the study and the experimental design. S-F. H. was involved in laboratory experiments and data analysis. T-C. C. wrote the manuscript.
References
- Shukla A, Rasik AM, Jain GK, et al. In vitro and in vivo wound healing activity of asiaticoside isolated from Centella asiatica. J Ethnopharmacol. 1999;65(1):1-1.
- Peoples JJ, Trivedi MK, Branton A, et al. Skin rejuvenating effect of consciousness energy healing treatment based herbomineral formulation. J Plant Biol. 2017;2(3):77-87.
- Sung JH, Park SH, Seo DH, et al. Antioxidative and skin-whitening effect of an aqueous extract of Salicornia herbacea. Biosci Biotechnol Biochem. 2009;73(3):552-6.
- Pain S, Altobelli C, Boher A, et al. Surface rejuvenating effect of Achillea millefolium extract. J Cosmet Sci. 2011;33(6):535-42.
- He ZQ, Findlay JA. Constituents of Astragalus membranaceus. J Nat Prod. 1991;54(3):810-5.
- Block KI, Mead MN. Immune system effects of echinacea, ginseng, and astragalus: a review. Integr Cancer Ther. 2003;2(3):247-67.
- Yesilada E, Bedir E, Çalış İ et al. Effects of triterpene saponins from Astragalus species on in vitro cytokine release. J Ethnopharmacol. 2005;96(1-2):71-7.
- Bedir E, Pugh N, Calis I, et al. Immunostimulatory effects of cycloartane-type triterpene glycosides from Astragalus species. Biol Pharm Bull. 2000 ;23(7):834-7.
- Zhang WD, Chen H, Zhang C, et al. Astragaloside IV from Astragalus membranaceus shows cardioprotection during myocardial ischemia in vivo and in vitro. Planta Med. 2006;72(01):4-8.
- Hashim P, Sidek H, Helan MH, et al. Triterpene composition and bioactivities of Centella asiatica. Molecules. 2011;16(2):1310-22.
- Sarkar D, Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006 ;236(1):13-23.
- Lee J, Jung E, Kim Y, et al. Asiaticoside induces human collagen I synthesis through TGFβ receptor I kinase (TβRI kinase)-independent Smad signaling. Planta Med. 2006;72(4):324-8.
- Kimura Y, Sumiyoshi M, Samukawa K-i, et al. Facilitating action of asiaticoside at low doses on burn wound repair and its mechanism. Eur J Pharmacol. 2008;584(2):415-423.
- Hsu LY, Nien CY, Huang WM, et al. Synthesis and protective effects of bis {4-[N, N-di-(carboxymethyl) amino] phenoxy} alkane derivatives on UVA-induced production of MMP-1 in human skin fibroblasts. Chem Pharm Bull. 2014;62(9):867-74.
- Henke H, Schenker TM, Cuénod M. Effects of retinal ablation on uptake of glutamate, glycine, GABA, proline and choline in pigeon tectum. J Neurochem. 1976;26(1):131-9.
- Fernandez-Patron C, Radomski MW, Davidge ST. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ Res. 1999;85(10):906-11.
- Burdick JA, Chung C, Jia X. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6(1):386-91.
- Osawa H, Kiwamoto T, Sherpa MT, et al. Hyaluronan Synthase 2 (HAS2) Mediates Elastase-Induced Airway Inflammation and Emphysema in Mice. Copd Basic Mechanisms 2020:A4757-A4757.
- Mikolajczyk P, Oberlander H, Silhacek DL, et al. Chitin synthesis in Spodoptera frugiperda wing imaginal discs: I. Chlorfluazuron, diflubenzuron, and teflubenzuron inhibit incorporation but not uptake of [14C] N‐acetyl‐D‐glucosamine. Arch Insect Biochem Physiol. 1994;25(3):245-258.
- Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266(5193):2011-5.
- Gold MH, Goldman MP, Biron J. Efficacy of novel skin cream containing mixture of human growth factors and cytokines for skin rejuvenation. J Drugs Dermatol. 2007;6(2):197-201.
- Tracy LE, Minasian RA, Caterson EJ. Extracellular matrix and dermal fibroblast function in the healing wound. Adv Wound Care. 2016;5(3):119-36.
- Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem. 1999;274(31):21491-4.
- Caley MP, Martins VL, O'Toole EA et al. Metalloproteinases and wound healing. Adv Wound Care. 2015;4(4):225-34.
- Albaugh VL, Mukherjee K, Barbul A, et al. Proline precursors and collagen synthesis: biochemical challenges of nutrient supplementation and wound healing. J Nutr. 2017;147(11):2011-7.
- Bukhari SN, Roswandi NL, Waqas M, et al. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int J Biol Macromol. 2018;120:1682-95.
- Necas JB, Bartosikova L, Brauner P, et al. Hyaluronic acid (hyaluronan): A review. Vet med. 2008;53(8):397-411.
- Bissett DL. Glucosamine: An ingredient with skin and other benefits. J Cosmet Dermatol. 2006;5(4):309-15.