Research Article - Current Pediatric Research (2023) Volume 27, Issue 7
The outcome of mandibular distraction osteogenesis in infants with severe Pierre Robin sequence in Vietnam.
Dang Hoang Thom1*, Vu Ngoc Lam2, Tran Thiet Son3
1Department of Paediatrics, Vietnam National Children’s Hospital, Hanoi, Vietnam
2Department of Nursing, 108 Military Central Hospital, Hanoi, Vietnam
3Department of Plastic Reconstructive Aesthetic Surgery, Hanoi Medical University, Hanoi, Vietnam
- Corresponding Author:
- Dang Hoang Thom
Department of Paediatric,
Vietnam National Children’s Hospital,
Hanoi,
Vietnam
E-mail: drthomdh@gmail.com
Received: 30-Mar-2023, Manuscript No. AAJCP-23-93682; Editor assigned: 03-Apr-2023, AAJCP-23-93682 (PQ); Reviewed: 17-Apr-2023, QC No. AAJCP-23-93682; Revised: 24-Jul-2023, Manuscript No. AAJCP-23-93682 (R); Published: 03-Jul-2023, DOI:10.35841/aajcp.27.07.1933-1937
Citation: Thom DH, Lam VN, Son TT. The outcome of mandibular distraction osteogenesis in infants with severe Pierre Robin sequence in Vietnam. Curr Pediatr Res. 2023;27(07):1933-1937.
Keywords
Mandibular distraction osteogenesis, Outcomes, Pierre Robin sequence, GI anomalies, Tracheostomies
Introduction
PRS is a group of birth defects including small jaw and tongue, with or without cleft palate; can lead to airway obstruction at the base of the tongue. The incidence of PRS is quite low, ranging from 1 in 5000 to 1 in 7000 births in the US [1].
When the mandible is underdeveloped, it can result in glossoptosis, which then causes problems with eating, Sleep Disordered Breathing (SDB) and upper airway obstruction [2,3]. In infants with PRS, SDB, particularly in the form of Obstructive Sleep Apnea (OSA), is very common, with a prevalence ranging from 85% to 100%. OSA in babies is linked to numerous negative health outcomes, including failure to thrive, developmental and learning delays, cor pulmonale and even death [4]. A comprehensive retrospective evaluation revealed that the mortality rate for PRS was 16.7% [5].
MDO described by Molina, et al. in 1995, is a relatively new therapy option in young patients with PRS has been become the cost effective option in comparison to tracheostomy and tongue lip adhesion [6-8].
Many researcher have reported on the efficacy of MDO in alleviating airway obstruction in the PRS population by gradually extending the jaw and pushing the tongue base forward [9,10]. As a result, MDO can expand supraglottic airspace and frequently reduce upper airway congestion.
The effectiveness of distraction is generally evaluated by improvement in clinical examination or polysomnogram results, de-cannulation of tracheostomy, tracheostomy avoidance, reduction in mortality or changes in airway obstruction patterns [11,12]. By this research, we provide the short term experience of a single clinic in treating juvenile patients with PRS with mandibular lengthening technique using internal distraction device systems.
Materials and Methods
Study design
We performed the longitudinal study of 143 infants with aged of 1-12 months diagnosed. Here, PRS infants underwent MDO at the Vietnam national children’s hospital in the period of 2019 to 2021.
Our study was evaluated and approved by an institutional review board obtained from Hanoi Medical University.
We did not include children older than 12 months, lacking information in the medical records. Information on each subject was reviewed simultaneously by 2 independent otolaryngologists to unify the results.
The following criterias: Age older than 12 months, incomplete medical records, and lack of pre or post MDO PSG were excluded. The medical records were checked by 2 independent otolaryngologists. Especially, with 25% of medical records were reviewed by both with higher than 90% inter-rater reliability.
Preoperative evaluation
In the event, the continuous pulse oximetry in the prone position is unsuccessful. To circumvent the tongue base, interventions like modified nasopharyngeal tubes and supplemental oxygen are employed. We also continuously monitor each child’s feeding response. In case of necessity, early nasogastric feeding will be applied to help supplement nutrition through the gastrointestinal tract, helping children gain optimal weight.
In cases where the obstruction is not relieved, the sleep is not of the required quality and the weight is not growing well, we will consider the surgical option.
Therefore, the assessment of the airway in PRS is very important because it affects the decision making of treatment. All infants had bronchoscopy prior to distraction to ensure that their airways were correctly analyzed. Tracheomalacia or any other anomalies that could not be corrected with distraction were not observed in them.
Statistical analysis
Demographics, operation information, total distraction, consolidation duration, hospital stay, and length of follow up were all gathered. Also documented were postoperative problems and surgical outcomes.
Descriptive statistics were used to examine demographic data. The chi-squared test was used to assess categorical variables and P, 0.05 was used to determine statistical significance. A statistical examination was conducted to explore any significant links between complication risk and variables such as gender, age, the presence of other anomalies and tracheostomy.
Results
In a cohort of 143 infants with PRS, 109 (76,2%) needed airway related surgery either mandibular distraction osteogenesis or tracheostomy, either mandibular distraction osteogenesis or tracheostomy. Seventy thirds of the infants (66.9%) were eventually enrolled in the study because they met all critical criteria.
The average age of the patients at the time of the procedure was 50.1 ± 42.8 days (ranging from 2 to 230 days), with an average hospital stay period of 32.5 ± 17.2 days (ranging from 2 to 105 days). The mean follow-up period was 9.6 ± 3.4 months, ranging from 6 to 15 months, as shown in Table 1.
| Factor | Males (n=32) | Females (n=41) | Overall (n=73) |
|---|---|---|---|
| Age (days) | 54.6 ± 48.0 | 46.5 ± 38.6 | 50.1 ± 42.8 |
| Follow-up period (months) | 9.7 ± 3.3 | 9.5 ± 3.1 | 9.6 ± 3.4 |
| Hospital stay (days) | 32.9 ± 11.5 | 33.2 ± 20.7 | 32.5 ± 17.2 |
| Total distraction (mm) | 15.1 ± 2.5 | 14.8 ± 3.1 | 14.9 ± 2.6 |
| Consolidation period (days) | 96.3 ± 36.1 | 94.7 ± 22.3 | 95.4 ± 33.6 |
Table 1. Demographic data of the patients (age, follow-up, hospital stay, total distraction and consolidation period were calculated as mean ± SD).
Cardiovascular abnormalities (n=13, 17.8%) and gastroesophageal reflux disease (n=11, 15.1%) were the most prevalent anomalies associated with PRS (Table 2).
| Abnormalities | Males | Females | Overall |
|---|---|---|---|
| Cardiac anomalies | 7 | 6 | 13 |
| GERD | 5 | 6 | 11 |
| GI anomalies | 5 | 3 | 8 |
| Laryngomalacia | 4 | 3 | 7 |
| CNS anomalies | 1 | 4 | 5 |
| Other anomalies | 1 | 2 | 3 |
| Note: CNS: Central Nervous System; GERD: Gastroesophageal Reflux Disease; GI: Gastrointestinal. | |||
Table 2. Abnormalities occurring concurrently with PRS.
Other than MDO, several individuals had surgical treatments, the most frequently occurring of these were gastrostomy tube installation (n=47) and pre-distraction tracheostomy (n=14) (Table 3).
| Surgical intervention | Male | Female | Overall |
|---|---|---|---|
| Gastrostomy tube placement | 23 | 24 | 47 |
| Pre-distraction tracheostomy | 4 | 10 | 14 |
| Cleft palate repair | 3 | 5 | 8 |
| Nissen fundoplication | 2 | 3 | 5 |
Table 3. Surgical interventions other than mandibular distraction.
All patients had effective mandibular distraction, with significant advancement of the lower jaw. The MDO resulted in a change in occlusion from class II to class I, with 2 mm to 3 mm overcorrection resulting in class III. The mean distraction achieved with internal devices was 14.9 ± 2.6 mm (range 9.3 mm-18.2 mm), as shown in Table 1.
Al patients gained weight quicker after MDO except for the patients who had a Nasogastric tube (NG) preoperatively (n=47). They also were released on 100% oral feeds. The NG was withdrawn one month after the MDO was completed to ensure that patients accepted feeds without difficulty.
The overall complication rate was relatively low, only 30.1% (n=22). Among the 22 patients who experienced problems, 19 (26.0%) had soft tissue infection and 3 (4.1%) had recurrent symptoms of Obstructive Sleep Apnea (OSA) despite MDO being conducted successfully (Table 4).
| Complications | Male | Female | Overall |
|---|---|---|---|
| Infection | 8 | 11 | 19 |
| Postoperative Sleep Apnea (OSA) | 1 | 2 | 3 |
| Re-operation | 0 | 0 | 0 |
Table 4. Related complications.
The Table 4 showed that there were 16 patients (21.9%) had superficial infections occurred around the site of distraction on the arm, but they were treated conservatively without requiring removal of the hardware. Three patients (4.1%) had a cheek abscess due to deep surgical site infection, which was managed with drainage and irrigation and had no effect on the distraction outcome.
A PRS patient who had a tracheostomy during infancy underwent MDO at one year of age; resulting in successful correction of retrognathia approximately 18 mm were gained during distraction. However, the patient continued to exhibit symptoms of Obstructive Sleep Apnea (OSA) after the distraction procedure, which required the use of Continuous Positive Airway Pressure (CPAP) and delayed decannulation until the age of two.
Statistic result
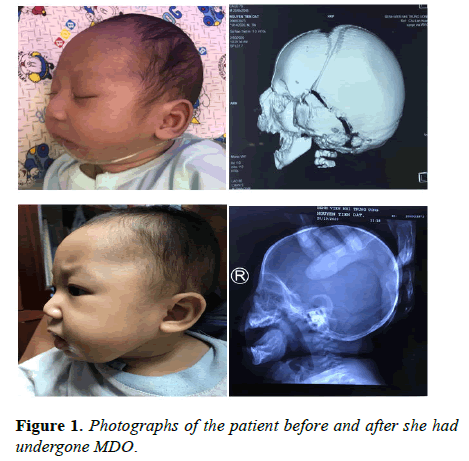
The frequency of complications was assessed by taking into account variables including gender, presented age, the presence of accompanying abnormalities such as cardiac anomalies and whether the patient had undergone tracheostomy prior to the procedure. Complication risk was not significantly connected with gender (P=0.514), age at presentation (P=0.935), existence of additional anomalies (P=0.822) or presence of tracheostomy (P=0.896), according to statistical analysis (Figure 1).
Discussion
MDO is a successful treatment for infants with PRS who experience significant airway obstruction. Additionally, the use of external distractors for MDO has been extensively explored as reported. The key advantages of these devices are that they do not require a second surgery to remove and that they can travel in multiple directions. However, significant downsides have been documented, including pin-site facial scarring, patient pain and greater exposure of the device to external damage, which may result in pin loosening/dislodgement, reduction of the device’s retention time and higher recurrence. The utilization of internal distractors in MDO has gained popularity due to its advantages, such as increased stability as it is not exposed to external trauma forces. Furthermore, utilizing a longer consolidation period after the procedure allows for improved ossification and a reduced risk of relapse. Additionally, the resulting scar in the submandibular region is considered more aesthetically pleasing [13,14].
A 9 years retrospective study conducted by CK Koustad, et al. recommended the use of MDO in infants to treat severe upper airway blockage and avoid the need for tracheostomy [15]. Infants with mild PRS are managed conservatively through patient positioning and nasal cannula oxygen supplementation, while those with severe PRS are intubated and scheduled for distraction within the same week. Brian T Andrews, et al. found that 51.7% of undistracted patients had other airway defects (e.g. subglottic stenosis, tracheomalacia) that would likely impact their distraction outcomes if they had not been pre-screened [16].
Denny AD, et al. recommend distracting the mandible until the maxillary and mandibular alveolar crests are well aligned, while Senders CW, et al. suggest distracting the mandible until it projects 2 mm to 3 mm beyond the maxillary alveolar ridge to allow for future bony relapse [17]. Senders CW, et al. also caution against over distraction as it may lead to future bony relapse or differential maxillary to mandibular growth. In this study, distraction was continued until class III was achieved, with the goal of significantly increasing the retroglossal airway and preventing future relapse [18].
Flores RL, et al. in a retrospective, database driven study found that the fewer days a child was treated, the better the outcome [19]. Infants with PRS who underwent distraction before 30 days of age were found to have a higher success rate in terms of respiratory status than those older than 2 months. This suggests that physiological changes may become entrenched and irreversible beyond a certain age, even with intervention.
Similarly Tahiri, et al. reported a higher complication risk in patients who were distracted at an older age. The mean age of patients who suffered issues in their study was 36.9 months, which was almost 1.5 years older than the mean age of all patients.
In this case, three (4.1%) had a tracheostomy at an age older than 3 years. Despite successful decannulation following MDO, two of these cases had unsatisfactory outcomes, with one requiring additional distraction and the other needing CPAP for chronic OSA. The statistical analysis did not indicate a significant correlation between patient age at presentation and the risk of complications.
Similarly, the presence of a tracheostomy did not increase the risk of complications in MDO for PRS, as identified by Flores, et al. Previous studies on MDO did not focus on its limitations in treating individuals with PRS. However, Flores, et al identified several factors that could predict the likelihood of MDO failure in PRS patients, including GORD, age older than 30 days, neurologic abnormalities, airway anomalies other than laryngomalacia, intact palate and pre-operative intubation. In this study, statistical analysis did not reveal any factors that increased the risk of complications.
Tholpady, et al shared their findings on the use of mandibular distraction in infants with PRS and concomitant laryngomalacia. They found that 23% of new-borns with PRS developed laryngomalacia, which required MDO. The authors suggest that lengthening the anterior peri-laryngeal tissue, including the extrinsic suprahyoid laryngeal muscles, enhances the diameter and flexibility of the airway. As a result, this corrects laryngomalacia and glossoptosis and avoids the need for tracheostomy [20]. This was also observed in our investigation, since we documented two cases of PRS with contemporaneous laryngomalacia. With the improvement of airway blockage, one of them was able to avoid tracheostomy. De-cannulation was successful in the second instance, which had previously been tracheostomized. None of them displayed any recurrence of airway blockage that necessitated intervention during the follow-up period.
Concerning feeding after MDO in PRS infants, studies have shown a considerable improvement in swallowing function after MDO [21,22]. As a result, the infants can feed orally when they are weaned from NG tubes pre-operatively. Hong, et al. found that MDO improved eating and swallowing performance in all of their PRS infants, which was validated by video fluoroscopic swallow investigations. In the current study, feeding was improved in all infants, including those who had a preoperatively implanted NG and were weaned after MDO.
There are some limitations to this study that should be considered. Although the population of the study is limited in absolute terms, the rarity of PRS makes it difficult to collect a large number of infants at a hospital. Furthermore, all of the infants included had severe airway disease, which may restrict the general application of our findings to PRS infants with a more typical distribution of airway severity, as well as those offering alternative procedures such as tongue-lip adhesion.
Conclusion
Using MDO in PRS is an effective technique to avoid future airway issues. Infants who required a tracheotomy prior to distraction and situations where distraction was performed at an older age (>2 months) had a lower success rate and a higher rate of complications. Laryngomalacia, gastric reflux disease, cardiac abnormalities and GI anomalies are not related with increased MDO failure rates in PRS.
Conflicts of Interest
The authors have no conflicts of interest, financial or otherwise.
References
- Scott AR, Mader NS. Regional variations in the presentation and surgical management of Pierre Robin sequence. Laryngoscope. 2014;124(12):2818-25.
[Crossref] [Google Scholar] [PubMed]
- Daniel M, Bailey S, Walker K, et al. Airway, feeding and growth in infants with Robin sequence and sleep apnoea. Int J Pediatr Otorhinolaryngol. 2013;77(4):499-503.
[Crossref] [Google Scholar] [PubMed]
- Anderson ICW, Sedaghat AR, McGinley BM, et al. Prevalence and severity of obstructive sleep apnea and snoring in infants with Pierre Robin sequence. Cleft Palate Craniofac J. 2011;48(5):614-8.
[Crossref] [Google Scholar] [PubMed]
- Singer LP, Saenger P. Complications of pediatric obstructive sleep apnea. Otolaryngol Clin North Am. 1990;23(4):665-76.
- Costa MA, Tu MM, Murage KP, et al. Robin sequence: Mortality, causes of death and clinical outcomes. Plast Reconstr Surg. 2014;134(4):738-45.
[Crossref] [Google Scholar] [PubMed]
- Molina F, Ortiz Monasterio F. Mandibular elongation and remodeling by distraction: A farewell to major osteotomies. Plast Reconstr Surg. 1995;96(4):825-40.
[Google Scholar] [PubMed]
- Molina F. Mandibular distraction osteogenesis: A clinical experience of the last 17 years. J Craniofac Surg. 2009;20 Suppl 2:1794-800.
[Crossref] [Google Scholar] [PubMed]
- McCarthy JG, Katzen JT, Hopper R, et al. The first decade of mandibular distraction: Lessons we have learned. Plast Reconstr Surg. 2002;110(7):1704-13.
[Crossref] [Google Scholar] [PubMed]
- Denny AD. Distraction osteogenesis in Pierre Robin neonates with airway obstruction. Clin Plast Surg. 2004;31(2):221-9.
[Crossref] [Google Scholar] [PubMed]
- Dauria D, Marsh JL. Mandibular distraction osteogenesis for Pierre Robin sequence: What percentage of neonates need it?. J Craniofac Surg. 2008;19(5):1237-43.
[Crossref] [Google Scholar] [PubMed]
- Flores RL, Tholpady SS, Sati S, et al. The surgical correction of Pierre Robin sequence: Mandibular distraction osteogenesis versus tongue-lip adhesion. Plast Reconstr Surg. 2014;133(6):1433-9.
[Crossref] [Google Scholar] [PubMed]
- Tahiri Y, Viezel-Mathieu A, Aldekhayel S, et al. The effectiveness of mandibular distraction in improving airway obstruction in the pediatric population. Plast Reconstr Surg. 2014;133(3):352e-9e.
[Crossref] [Google Scholar] [PubMed]
- Hong P. A clinical narrative review of mandibular distraction osteogenesis in neonates with Pierre Robin sequence. Int J Pediatr Otorhinolaryngol. 2011;75(8):985-91.
[Crossref] [Google Scholar] [PubMed]
- Genecov DG, Barcelo CR, Steinberg D, et al. Clinical experience with the application of distraction osteogenesis for airway obstruction. J Craniofac Surg. 2009;20 Suppl 2:1817-21.
[Crossref] [Google Scholar] [PubMed]
- Kolstad CK, Senders CW, Rubinstein BK, et al. Mandibular distraction osteogenesis: At what age to proceed. Int J Pediatr Otorhinolaryngol. 2011;75(11):1380-4.
[Crossref] [Google Scholar] [PubMed]
- Andrews BT, Bradley JP. Reply: Incidence of concomitant airway anomalies when using the University of California, Los Angeles, protocol for neonatal mandibular distraction. Plast Reconstr Surg. 2013;132(6):1072e-3e.
[Crossref] [Google Scholar] [PubMed]
- Denny AD, Talisman R, Hanson PR, et al. Mandibular distraction osteogenesis in very young patients to correct airway obstruction. Plast Reconstr Surg. 2001;108(2):302-11.
[Crossref] [Google Scholar] [PubMed]
- Senders CW, Kolstad CK, Tollefson TT, et al. Mandibular distraction osteogenesis used to treat upper airway obstruction. Arch Facial Plast Surg. 2010;12(1):11-5.
[Crossref] [Google Scholar] [PubMed]
- Flores RL, Greathouse ST, Costa M, et al. Defining failure and its predictors in mandibular distraction for Robin sequence. J Craniomaxillofac Surg. 2015;43(8):1614-9.
[Crossref] [Google Scholar] [PubMed]
- Tholpady SS, Costa M, Hadad I, et al. Mandibular distraction for Robin sequence associated with laryngomalacia. J Craniofac Surg. 2015;26(3):826-30.
[Crossref] [Google Scholar] [PubMed]
- Hong P, Brake MK, Cavanagh JP, et al. Feeding and mandibular distraction osteogenesis in children with Pierre Robin sequence: A case series of functional outcomes. Int J Pediatr Otorhinolaryngol. 2012;76(3):414-8.
[Crossref] [Google Scholar] [PubMed]
- Breik O, Umapathysivam K, Tivey D, et al. Feeding and reflux in children after mandibular distraction osteogenesis for micrognathia: A systematic review. Int J Pediatr Otorhinolaryngol. 2016;85:128-35.
[Crossref] [Google Scholar] [PubMed]