- Biomedical Research (2016) Volume 27, Issue 3
The neuro-protective effects of water extract of Faces Trogopterori on transient MCAO rats.
Qiaoling Zhai*
Department of Medical Examination, People’s Hospital of Zhengzhou, PR China
- Corresponding Author:
- Prof. Qiaoling Zhai
Department of Medical Examination People’s Hospital of Zhengzhou PR China
E-mail: qiaolingzhai@sina.com
Accepted date: April 3, 2016
Abstract
Objective: To get the water extracts of Faeces Trogopterori (WEFTs), and study its effect on Neurological function, inflammatory reaction and oxidative damage caused by ischemia-reperfusion injury of transient MCAO SD rats.\
Method: Neurological evaluation was conducted by modified Longa score, infarction volume percentage was calculated by TTC staining method and a normal method which has exclude the error of edema; SOD activity was tested by xanthine oxidase method, MDA content was determinate by TBA (Thiobarbituric acid) method, IL1-β level in brain tissue and serum in the ischemia-reperfusion rats was got by Elisa.
Result: There was no statistical difference between the score Faeces Trogopterori Groups and control group, but significant statistical difference there was between the two groups, infarction volume ratio of extracts groups was 35%, while the control group at 50%; Faeces Trogopterori group could significantly reduce the MDA content (p<0.01, vs model group), increased SOD activity (p<0.01, vs. model group).
Conclusion: Aqueous extract of Faeces Trogopterori could exert certain protective effect on ischemiareperfusion injury caused by MCAO rats.
Keywords
MCAO, Faeces Trogopterori, Neuroprotective, IL1-β, Injury of reperfusion.
Introduction
Faeces Trogopterori is a kind of traditional Chinese medicine, it has many active compounds like nitrogen, triterpene, phenolic acids, microelement, volatile components. Its pharmacological activities are included cytotoxic effect, antiinflammatory, anti-ulcer, the effect on the blood system and other pharmacological activities. For triterpene composition of Faeces Trogopterori., 7 kinds of terpene has cytotoxic effect, especially for the effect on the P-388 which is a kind of granulocyte leukemia [1] found that water decoction of Faeces Trogopterori can obviously improve the function of T cells lymphocyte leansformstoin; higher Ts caused by ALS (immune lymphocytes in mice serum) return to normal, improve immune function of mice injected with ALS [2]. Wang et al. make the study of ethyl acetate extract from Faeces Trogopterori, found that this extracts could significantly reduce the content of prostaglandin E (PGE) among the inflammation tissue, but had no significant effect on serum corticosterone level, shows that its anti-inflammatory effects may be associated with inhibition of the synthesis and release of PGE [2,3].
We know that many traditional Chinese medicines (TCMs) could activate blood circulation and improve hemorheology, anti-platelet aggregation and so on anticoagulant effect. Faeces Trogopterori is the one we mentioned medicine above, has fibrinolytic function, can produce the fibrinolysis against urokinase [4]. WEFTs can obviously inhibit the rabbit platelet aggregation induced by ADP, collagen, its inhibitory effect is related with the dose, and intraperitoneal injection into the SD rats could also get the similar results as the above. Intravenous injection in rat had obvious inhibitory effect on perimental thrombosis in carotid artery-venous bypass [5]. WEFTs can significantly prolong the survival of incomplete cerebral ischemia mice, and can reduce ischemic tissue MDA content, improve the activity of superoxide dismutase (SOD), shows that Faeces Trogopterori has certain protective effect in the MCAO model of mice [6,7]. Chen et al. reported that ethyl acetate extract of Faeces Trogopterori has similar contents as the Luoxintong L2 (rutin), thus may have similar effects as the latter [8]. Therefore, according to all these foundation, Faeces Trogopterori has great potential on the treatment of ischemic diseases of the brain. Up to now, no one has studied the effect of WEFTs on the influence of neurofunction in the ischemic brain damage SD rats.
Methods
Preparation of WEFTs [9]
Accurately weight 500 g Faeces. Trogopterori., added 1000 ml water and soaked for 1 h; decoction for 30 min with high heat, gentle heated for 30 min, filtered for filtrate; filtration residue was added 1000 ml of water and gentle heated for 1 h, got the filtrate; Merge the two filtrate, give slow fire decoction and concentration, at last filtrate got 500 ml (1 ml solution is technical 1 g) 4 stored.
MCAO model [10]
The protocol we used modified Zea Longa method, and specific operation was as followed: weight the rat, induction anesthesia with 5% isoflurane, 1.5% ~ 2% isoflurane anaesthesia. Take the neck midline incision, CCA and ICA were clipped temporarily, insert the suture line from the snip in ECA, slowly pushed into the ICA in cranial artery branch, loosen the ICA artery clamp, ligation suture. The depth from the top and ECA bifurcation about 20 mm, the suture was ligated at the CCA bifurcate, skin sutured. Ischemia after 2 h, pull out the suture, after ECA was electric coagulated, suture the incision.
Grouping and administration [11]
40 healthy male SD rats, weighted from 280-320 g, by laboratory animal center of school of medicine in Tongji University. All SD rats were randomly divided into four groups named: Low dose Group of Faeces. Trogopterori. (n=8), High dose Group of Faeces. Trogopterori. (n=8), model group (n=8), Sham group (n=8), ligustrazine (Lig, ligustrazine) Group (n=8). Including Low dose concentration was 20 mg/kg, High dose concentration was 60 mg/kg, ligustrazine concentration was 20 mg/kg. Rats in Model group only received MCAO treatment and taken 3mL physiological saline; Sham group taken the same treatment as model group rats, but no insert with suture, taken 3mL physiological saline. Drug taken for 7 d by i.p. Injection.
Neurological evaluation [12]
Neurological evaluation was performed when the animals were allowed 24 hours later from the MCAO model was finished. Scored on a 6-point scale as described previously (0=no neurological deficit, 1=failure to extend right for paw fully, 2=circling to the right, 3=falling to the right, 4=no spontaneous walking with depressed level of consciousness and 5=death). In the suture model, animals were excluded for the following criteria: score lower than 1 point, subarachnoid hemorrhage, and death.
TTC assessment [13]
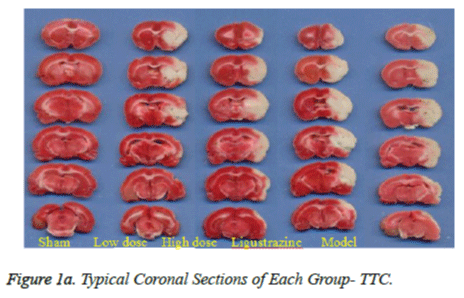
To analyze the infarct volume, the rats were euthanized with pentobarbital sodium at the end of the drug infusion, and their intact brains were quickly removed and sectioned into 2 mm thick slices starting at the frontal pole. The slices were then immersed in the vital dye (2%) 2,3,5- triphenyltetrazoliumchloride (TTC; Sigma Aldrich Corp., USA) in a painting box for 5 min, and the slices were turned over again to obtain consistent dyeing of the anterior and posterior faces, then put it into a 4% formaldehyde solution, fixation at 4. We got the colour pictures by scanning these slices and in this process put a ruler on the scanner, in order to calculate infarct size.
Briefly, the infarct volume was calculated by summing the infarct areas of all sections and multiplied by the slice thickness; the infarct area in the ipsilateral hemisphere was indirectly measured by subtracting the non-infarct area in the ipsilateral hemisphere from the total intact area of the contralateral hemisphere. All brain slices were analyzed for the infarct volume by Image J software. SOD activity assay.
SOD activity assay [14]
SOD vitality was tested by xanthine oxidase method. Following kit instructions, absorbance was measured at 550 nm with spectrophotometer, myocardial tissue SOD activity was calculated according to the following formula:
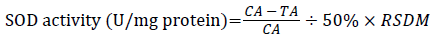
÷ TP (mg protein/ml)
CA: Absorbance in the Control Tube; TA: Test Absorbance in the Sample Tube; RSDM: Dilution Multiple Reaction System; TP: Tissue Protein Content.
MDA content
Lipid peroxide degradation products MDA content was detected by glucosinolates barbituric acid (TAB) condensation method determination. According to the kit instructions, absorbance was determined at 532 nm. Brain tissue MDA content in the according to the following formula:
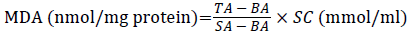
÷ TP (mg protein/ml)
TA: Test Absorbance; SA: Standard Absorbance; BA: Blank Absorbance; SC: Standard Concentration; TP: Tissue Protein Content.
IL-1β determination in brain and serum [15]
IL-1β content was tested by ELISA. Antibody of detection phase was polyclonal antibody biotin labeled. Samples and biotin labeled antibody were added into plate hole in sequence, PBS washed. Peroxidase labeled avidin was added, PBS was washed thoroughly, the substrate color was observed by TMB color, then changed into blue, eventually translate into yellow. According to the kit instructions, draw standard curve and calculate the sample concentration, added diluted standard sample and sample liquids into the corresponding enzyme label plate hole, incubated at 37 for 90 min, then washed the plate added into each hole with 1:200 second antibody 0.1 mL, incubated for 60 min at 37; then washed and 0.1 ml 1:600 enzyme-linked was added, 37 for 30 min; After washed and 0.1 mL substrate was added, in dark for 15 min, then 0.05 mL termination liquid was added, absorbance at 450 mm was measured, the calculation of IL-1β in brain tissue and serum (pg/ml).
Results
Neurological evaluation
Twenty-four hours after the onset of MCAO, neurological deficit scores in MCAO rats were significantly higher (p<0.01) than control ones (Table 1), indicating that the MCAO model had effectively established cerebral ischemia. As compared to the model control group, after the treatments with 20 mg/kg and 60 mg/kg WEFTs, the neurological deficit scores significantly decreased.
| Group | Dose(mg/kg) | Neurological evaluation |
| Sham | Saline | 0.10 ± 0.18** |
| Model | Saline | 2.12 ± 0.38 |
| Low dose | 20 | 1.72 ± 0.41 |
| High dose | 60 | 1.25 ± 0.26* |
| Ligustrazine | 20 | 0.72 ± 0.13** |
*p<0.05 represents a significant difference between control group and other groups.
**p<0.01 represents a significant difference between control group and other
groups.
Table 1. The Effects of WEFTs on neurological scores of rats after reperfusion for 24 h (n=10).
Infarction volume assessment
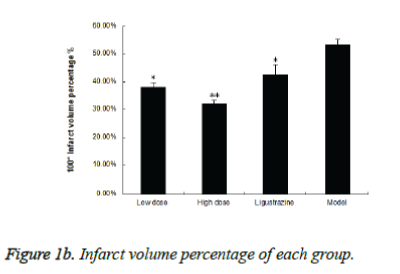
Figure 1a shows typical images of the coronal sections from each group. Treatments with two groups of WEFTs significantly reduced the infarct volume and showed dosedependent reductions compared with the control group. Infarct volumes after treatments with 20 or 60 mg/kg of WEFTs were reduced approximately by 15.3%, 21.3% ( Figure 1b ), versus the control group ( control: 53.4 ± 2.1%; 20 mg/kg WEFTs, 40.6 ± 1.60%; 60 mg/kg WEFTs, 10.68 ± 1.2%; 20.0 mg/kg ligustrazine, 42.6 ± 3.50% ). The protective effects of WEFTs at 20 mg/kg could exert a little bigger effect as the ligustrazine group.
The effects on the SOD and MDA in brain tissue
With the acute cerebral ischemia-reperfusion injury, the SOD activity in the model group was greatly decreased when compared with the Sham group (p<0.01), and the MDA content was increased significantly (p<0.01). When WEFTst group was compared with model, all the three groups exhibited increased SOD activity obviously (p<0.01 or p<0.05), extract group were better; While the MDA content decreased significantly in all the three groups (p<0.01), and no significant difference between the two WEFTs (p>0.05), (Table 2).
| Group | Dose (mg/kg) | SOD (U/mg prot) | MDA (nmol/mg prot) |
| Model | - | 81.1 ± 6.7## | 4.78 ± 0.67## |
| Sham | - | 121.2±5.8 ** | 1.72±0.15 ** |
| Low dose Group | 20 | 108.7±8.1 ** | 3.15±0.12 ** |
| High dose Group | 60 | 112.5±9.2 ** | 3.63±0.21 ** |
| Ligustrazine | 20 | 90.7±7.5 * | 2.58±0.14 * |
Note: ## p<0.01 vs Sham; *p<0.05, ** p<0.01 vs Model.
Table 2. Effects on the SOD levels and MDA contents (x ± s, n=8).
IL-1β level in brain tissue and serum
Compared with the Sham group, IL-1β level in Model group showed significantly increased (P<0.05). When the results of ligustrazine group rats were compared with Model group, the WEFTs could be significantly reduced in the brain tissue and serum (P<0.01), but were slightly weaker than ligustrazine group (Table 3).
| Group | Dose (mg/kg) | Cerebrum IL-1β (pg/ml) | blood serum Il-1β (pg/ml) |
| Model | - | 1015.2±247.1## | 379.1±76.6# |
| Sham | - | 629.8±117.2 | 256.4±53.8 |
| Low dose Group | 60 | 995.3±124.7 | 340.6±75.2 |
| High dose Group | 200 | 550.7±99.8** | 278.5±62.1** |
| Ligustrazine | 60 | 480.7±114.5** | 262.9±90.8** |
Table 3. Effects of WEFTs on the IL-1β levels in brain tissue and serum (x±s, n=8)
Discussion
To set up an ideal animal model of cerebral ischemia is one of the important tools to study the pathophysiological changes of cerebral ischemia and to evaluate means of effective intervention measures. Because the cerebral vascular anatomy and the function of the rat are similar as human, therefore it is generally believed that the rat is the most suitable animal. We chose the male SD rats as the cerebral ischemia model animal, and conduct the MCAO model. At present, the preparations of the focal cerebral ischemia model are various. However, each of them has their own advantage and disadvantage [16]. For Monofilament nylon suture made MCAO model, the process of preparation is simple, do not need to expose MCA craniotomy, light damage to animals, small impact on the whole body, the success rate is relatively high, not cause big changes of blood pressure, blood gas and the temperature changes after occlusion of MCA. Cause not craniotomy and small trauma, not affect the pathological changes of brain edema and intracranial pressure after ischemic, it is better than that of ligation MCA method with craniotomy; At the same time avoid the embolus randomness brought by internal carotid artery injection occluded MCA, it is especially suitable for focal cerebral ischemia-reperfusion injury of research; Monofilament nylon suture method could block artery of cerebral hemorrhage and the branch vessel of cortical from the MCA that could make relatively occlusion effect [17].
Prone position of ischemic stroke is in the brain arteries, the arteries occlusion lead to the formation of focal cerebral ischemia, its residual cerebral blood flow was rare due to central ischemia, generally the development of irreversible brain damage and hasten death no more than 1 hour. But there are still a small amount of blood in the surrounding brain tissue ischemia central area, could form reversible ischemic injury, still make a balance of ion and cellular structure integrity, this kind of tissue could be known as the ischemic penumbra [18]. If blood flow is restored and drug intervention is given as soon as possible, that would greatly restore the function of ischemic brain tissue, relieve cerebral edema, improve neurologic deficits, and decrease infarct volume.
Our results show that compared with sham group, model group showed significant neuron-function defect, has obvious infarcts, these indicators show that model is successful. The above features and morphological index were all improved by the WEFTs groups, and its effect was similar to the positive medicine ligustrazine, thus the Faeces. Trogopterori. We mentioned above could protect neurons, delay the death of nerve cells, and maintain the structure and permeability of blood brain barrier, reduce ischemia - reperfusion injury.
References
- Numata A, Yang P, Takahashi C, Fujiki R, Nabae M, Fujita E. CytoxicTriterpenes from a Chinese Medicine. Chem Pharm Bull1989; 37: 648.
- Tang X. Effects of Faeces. Trogopteri on cells of atherosclerotic rats intercellular adhesion molecule-1 Third Military Medical University 2009.
- Wang S, Liu Y, Song L, Wang M, Zhu Y, Wang A. Pharmacological studies on anti-platelet aggregation effect of Faeces. Trogopteri. Journal of Shenyang College of medicine 1994; 04: 246-250.
- Yu Z. Dissolution effects of 21 kinds of traditional Chinese medicine on the in-vitro fibrinolytic. J Integr Med 1986; 08:484-485.
- Wang S, Song L, Liu Y, Zhang X, Xu X, Yu L. Anti-inflammatory effects of ethyl acetate extract of Faeces. Trogopterori. J Shenyang Col Med 1994; 01: 49-53.
- Yuekai C, Haixiong W, Qinsheng Y. Effect of Faeces. Trogopterion the activity of SOD in rat blood. Chinese J of BiochemPharmaceut 1994; 15(03): 161-164.
- Shu-min BU,Mao Hua J, Yun-bo Q. Protective effects of Faeces. Trogopterion cerebral ischemia in mice and rats. J of Shanxi Univ 23: 257-259.
- Yuekai C, Shaosong C, Meisu W. Study on the flavonoids of Faeces. Trogopteri. J of Shanxi Univ 2005; 28: 98-100.
- Liu W, Li Q, Deng Y, Cheng Z.Study on the inhibition effect of faeces Trogopterori extract on gastric acid secretion of rats and its chemical constituents. Chinese J of Trad Med Sci and Technol 2003; 03:176.
- Zhang R, Chopp M, Chen H, Garcia JH. T emporal profile of ischemic tissue damage, neutrophil response, and vascular plugging following permanent and transient (2H) middle cerebral artery occlusion in the rat. J NeurolSci1994; 125: 3-10.
- Liang H, Liu P, Wang Y, Song S, Ji A. Protective effects of alkaloid extract from Leonurusheterophyllus on cerebral ischemia reperfusion injury by middle cerebral ischemic injury (MCAO) in rats. Phytomedicine2011; 18: 811-818.
- Girgis H, Palmier B, Croci N, Soustrat M, Plotkine M, Marchand-Leroux C. Effects of selective and non-selective cyclooxygenase inhibition against neurological deficit and brain oedema following closed head injury in mice. Brain Res 2013; 1491: 78-87.
- Kramer M, Dang J, Baertling F, Denecke B, Clarner T, Kirsch C, Beyer C, Kipp M. TTC staining of damaged brain areas after MCA occlusion in the rat does not constrict quantitative gene and protein analyses. J Neurosci Methods 2010; 187: 84-89.
- Liu H, Zhang X, Du Y, Ji H, Li S, Li L, Xing Y, Zhang X, Dong L, Wang C, Zhao K, Ji Y, Cao X.Leonurine protects brain injury by increased activities of UCP4, SOD, CAT and Bcl-2, decreased levels of MDA and Bax, and ameliorated ultrastructure of mitochondria in experimental stroke. Brain Res2012; 1474: 73-81.
- Amantea D, Certo M, Russo R, Bagetta G, Corasaniti MT, Tassorelli C. Early reperfusion injury is associated to MMP2 and IL-1β elevation in cortical neurons of rats subjected to middle cerebral artery occlusion. Neuroscience 2014; 277: 755-763.
- Yanamoto H, Nagata I, Niitsu Y, Xue JH, Zhang Z, Kikuchi H.Evaluation of MCAO stroke models in normotensive rats: standardized neocortical infarction by the 3VO technique. ExpNeurol2003; 182: 261-274.
- Shimamura N, Matchett G, Tsubokawa T, Ohkuma H, Zhang J.Comparison of silicon-coated nylon suture to plain nylon suture in the rat middle cerebral artery occlusion model. J Neurosci Methods 2006; 156: 161-165.
- Zhang X, Deguchi K, Yamashita T, Ohta Y, Shang J, Tian F, Liu N, Panin VL, Ikeda Y, Matsuura T, Abe K.Temporal and spatial differences of multiple protein expression in the ischemic penumbra after transient MCAO in rats. Brain Res2010; 1343: 143-152.