Research Article - Biomedical Research (2017) Volume 28, Issue 4
The modulatory effect of Schisandra chinensis seed extract on cisplatin induced cytotoxicity and cell proliferation inhibition in human osteosarcoma cells
Li Yin*, Fenglin Zhong, Chenyang Zhao, Shaojiang Liu and Heng XiaoDepartment of Orthopaedics, Panzhihua Central Hospital Sichuan, China
Accepted date: September 30, 2016
Abstract
Aim: To evaluate the effect of Schisandra chinensis seed extract (used in Chinese medicine) on cisplatininduced cytotoxicity in osteosarcoma cells.
Methods: MTT and caspase assays were used to determine the apoptotic potential of cisplatin and Schisandra chinensis seed extract on Human Osteosarcoma Cells (HOS). The effect of exposure to cisplatin and Schisandra chinensis extract on the expression of caspase 3 was evaluated. The sensitivity of osteosarcoma cells to cisplatin and the expression of phosphor glycoprotein and cisplatin separately and in combination with Schisandra chinensis extract were assessed.
Results: The results of the study indicate cisplatin and seed extract of Schisandra chinensis induced apoptosis in human osteosarcoma cells. The combination of cisplatin and seed extract produced a significant increase in the apoptosis level in osteosarcoma cells. Caspase activation increased significantly on treatment with cisplatin and of Schisandra chinensis seed extract. The seed extract increased the cisplatin sensitivity as well as the expression of phosphoglycoprotein.
Conclusion: Schisandra chinensis seed extract ameliorates cisplatin-induced cytotoxicity through apoptosis and increases sensitivity by increasing the expression of p-glycoprotein.
Keywords
Cisplatin, Schisandra chinensis, Osteosarcoma, Cytotoxicity
Introduction
Osteosarcoma is a disease that affects people of all ages all over the world. The disadvantage of using chemotherapeutic combinations is that resistance can develop [1]. Thus there is a need for better chemotherapeutic regimens.
Cisplatin is a platinum co-ordination complex used as a chemotherapeutic agent in ovary, testicular, breast, lung and colon cancer [2]. Intercalation of DNA by cisplatin induces apoptotic cell death. Cisplatin is a better regimen for decreasing chemotherapeutic resistance because it is cytotoxic in osteosarcoma cells [3-5]. Cisplatin has limitations of nephrotoxicity and leucopenia, which are dose limiting. They also limit the therapeutic range of cisplatin. Carboplatin is in 2-4 higher concentration sensitive as cisplatin in osteosarcoma cells. In cisplatin therapy intracellular drug accumulation is an important determinant of CDDP-resistance, which compromises therapeutic efficacy [6].
The genus Schisandra (family Magnoliaceae) consists of 25 species. Schisandra chinensis is also known as Fructus schisandra or five-flavour berry. It is reported to be have antioxidant, anti-stress, hepatoprotective, endocrine, antiinflammatory and anti-mutagenic properties. Schisandra chinensis seed extract contains the active principle schisandrin. This traditional medicine of the Chinese Pharmacopoeia was officially used as a medicine in the USSR decades before. Western scientists have not studied the use of this substance. Recently, the active principle of Schisandra chinensis, schisandrin, has been described as a potential MDR-reversing agent in chemotherapy for osteosarcoma [7,8].
In this investigation, we studied the effect of Schisandra chinensis seed extract on cisplatin-induced cytotoxicity, apoptosis and cisplatin sensitivity in human osteosarcoma cells. Apoptotic activity was verified through the activation of caspase-3 signalling by cisplatin and Schisandra chinensis seed extract [9-12]. The effect the cisplatin and Schisandra chinensis seed extract on p-glycoprotein expressional level might ameliorated cisplatin-induced cytotoxicity in osteosarcoma cells.
Materials and Method
Cell line, cell culture and treatment
Human osteosarcoma cells (1547) were obtained from ATCC. The cell lines were trypsinised and cultured, and the cells were counted on a haemocytometer. The cell viability was determined using trypan blue. Freshly trypsinized cells were seeded at 4 × 105 cells/cm2 on Eagle’s essential medium (GibcoBRL, Cergy-Pontoise, France) supplemented with 10% Foetal Calf Serum (FCS) (GibcoBRL) and 100 U/ml penicillin. The cultures were maintained in a humidified atmosphere with 5% CO2 at 37°C. The cells were grown up to 90% confluence otherwise reseeded. The same amount of drug vehicle ethanol was added to control group, in which the cells not being treated with drugs.
Cell proliferation assay
The MTT assay was used to determine cell proliferation using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide. In this assay, human osteosarcoma cells were trypsinised after attaining confluence in 96-well plates in triplet for each drug treatment. The cells were treated with ethanol, cisplatin, Schisandra chinensis seed extract and both cisplatin and seed extract.
Quantification of apoptosis
Human 1547 osteosarcoma cells were seeded in six-well culture plates. After treatment with the drug (40 WM) for 12 hours, an increase in the proportion of floating cells was observed. The extent of apoptosis was measured through secondary apoptosis, quantified by the cell death marker caspase through ELISA (Cell Death Detection ELISA, Roche Diagnostics). The apoptosis assay was carried out with cytosol supernatant of the osteosarcoma cells.
Evaluation of cellular sensitivity to cisplatin
The cellular sensitivity to cisplatin and the effect of the seed extract on the sensitivity were determined by growth inhibition of cells at 50% of the population after 1 hour of drug exposure. CDDP (50 mM HEPES, pH 7.4, 100 mM NaCl, 0.1% CHAPS, 10 mM DTT, 1 mM EDTA and 10% glycerol) was added to each well of a microtiter plate and incubated at 37°C for 10 minutes. Finally, the substrate 1011 Ac-DEVDpNA was added. After 12 hours, the optical density at 405 nm was measured using a microtiter plate reader.
Resistance factor = (IC50 HOS 1547/Pt cells)/(IC50 HOS 1547 cells)
Western blot analysis
The western blot was used to understand the protein expression level changes with drug treatment. The osteosarcoma cells were washed twice with PBS, collected and lysed in a lysis buffer (100 mM Tris-HCl, pH 6.8, 4% w/v SDS, 1 mM phenylmethylsulfonyluoride, 200 mM b-mercaptoethanol and 1 g/ml aprotinin). The cell lysates were centrifuged at 12,000 g for 30 minutes at 4°C to obtain a cell membrane using the membrane protein extraction kit (Beyotime, Haimen, Jiangsu, China). The protocol recommended for the kit was followed. The protein concentration was determined using Bradford’s method. 10% SDS-PAGE was used to separate the protein by loading 20 μg. The protein was transferred onto PVDF membranes (Bio-Rad Inc., Hercules, CA, USA). Immune complexes were formed by incubating proteins with primary antibodies (1:1000 dilution) overnight at 4°C and then with secondary antibodies (1:10,000 dilution) for 2 hours at 25°C. After extensive washing with TBST, the membranes were processed with the Easy See Western Blot Kit (Trans Gen Biotech, Beijing, China) according to the manufacturer’s protocol. Immunoreactive protein bands were detected using a ChemiDoc XRS+ imaging system (Bio-Rad, Inc.).
Statistical analysis
The data were expressed as mean+SEM values, and the means of the groups were statistically compared by Analysis of Variance (ANOVA). A P-value less than 0.05 was considered statistically significant.
Results
Effect of drug treatment on cell growth
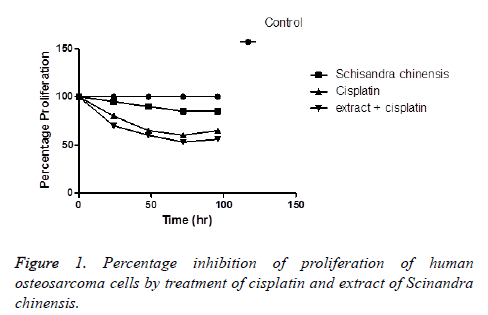
Human osteosarcoma cells with 10% foetal calf serum and DMEM with cisplatin or both cisplatin and seed extract were observed using the MTT assay. The cell proliferation was significantly inhibited with treatment with cisplatin and with both cisplatin and seed extract. The cell proliferation inhibition was expressed as the percentage inhibition (Figure 1).
Effect of seed extract on to cisplatin induced apoptosis
Cisplatin-induced cell death by intercalation with DNA causes inhibition of cell proliferation through apoptosis. Cell fractions were used with ELISA to determine apoptosis by floating cells compared with adherent cells. The cisplatin and seed extract were evaluated for apoptosis after 12 and 24 hours of treatment as shown in Table 1. There were significant increases in the numbers of floating cells after treatment with cisplatin and both seed extract and cisplatin. Moreover, the apoptotic ratio, determined by ELISA, increased significantly over time.
| Time | Control | Scinandra chinensis extract | Cisplatin | Scinandra chinensis extract+Cisplatin |
|---|---|---|---|---|
| 0 hour | 0 | 0 | 0 | 0 |
| 12 hour | 2.40 ± 1.1* | 3.3 ± 1.27* | 3.90 ± 1.4* | 4.2 ± 1.35* |
| 24 hour | 5.35 ± 1.3* | 5.9 ±1.42* | 6.4 ±1.54* | 6.9 ± 1.56* |
| All values are expressed as Mean ± S.E.M. where *P<0.05, **P<0.01 a vs. Control. | ||||
Table 1. Ratio of apoptotic cells in HOS cells.
Effect of seed extract on cellular sensitivity to cisplatin induced cytoxicity
A growth inhibition assay (IC50) was used to determine the cellular sensitivity to cisplatin and both seed extract and cisplatin after 1 hour of treatment. The IC50 values are shown in Table 2. Under our experimental conditions, the HOS 1547/Pt cell line was approximately 2-5 more resistant to CDDP.
| Drugs | IC50 (uM) | Resistance factor | |
|---|---|---|---|
| U2-OS | U2-OS/Pt | ||
| Cisplatin | 16.9 ± 4.8 | 84.8 ± 4.5 | 5.01 |
| Scinandra chinensis extract | 80.7 ± 4.5 | 94.6 ± 5.2 | 1.17 |
| Cisplatin+Scinandra chinensis extract | 18.4 ± 4.1 | 74.6 ± 4.9* | 4.05 |
Table 2. Effect of cisplatin and Scinandra chinensis extract on sensitivity to osteosarcoma cells U2OS and platinum resistance cells.
Effect of seed extract on caspase 3 expression and glycoprotein of cisplatin induced cytoxicity
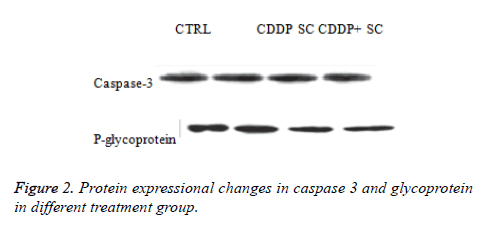
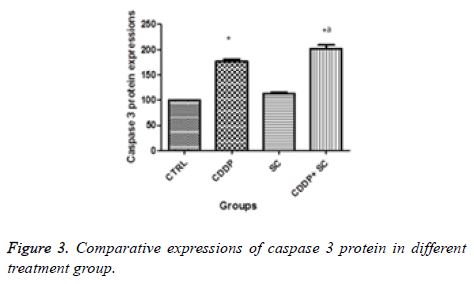
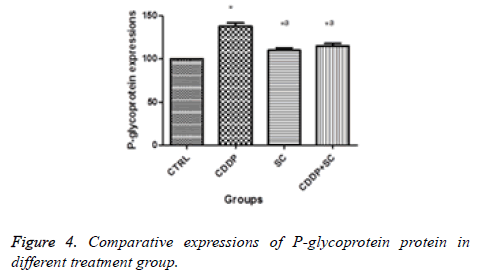
In the apoptosis induction mechanism, caspase 3 is activated during the signalling process. It has been reported that cisplatin induces apoptosis through caspase 3 activation in osteosarcoma cells. The activation of caspase 3 was increased significantly in the human osteosarcoma cells treated with cisplatin and seed extract. The reduction of the expression of glycoprotein was more significant with the cisplatin and seed extract treatment compared with cisplatin alone (Figures 2-4).
Discussion
Cisplatin induces cell death mostly because of intercalation with DNA, which results in apoptotic cell death and oxidative stress [13-15]. The active constituent, schinadrin, has various uses in Chinese traditional medicine which causes MDR resistance in cisplatin-treated osteosarcoma cells.
Schisandrin B provides protection from doxorubicin-induced cardio toxicity and increases anticancer activity by acting as a chemosensitiser in S180 sarcoma and 4T1 breast cancer. Deoxyschizandrin shows beneficial anticancer effects through its anti-proliferative activity in human U2OS osteosarcoma cells. Schinandrol, an active constituent of Schisandra parts, ameliorates acetoaminophen-induced hepatotoxicity through its antioxidant effect and activation of the NRF2–ARE signalling pathway, which is involved in xenobiotic detoxification. Schisandrin B is a mixture of diastereoisomengomisin N and γ- schisandrin. Whereas gomisin N inhibited ataxia telangiectasia and Rad-3-related protein kinase in a dose-dependent manner, whereas γ-schisandrin did not [16-18].
In the present experiment, Schisandra chinensis seed extract, with cisplatin, induced apoptosis and inhibited cell proliferation more effectively compared with cisplatin alone (Table 1). Further, caspase 3, a marker of apoptosis, was activated by cisplatin, and the effectiveness was better in combination with the seed extract (Table 2). In another study, cisplatin accumulation and sensitivity of cisplatin were not affected significantly by the seed extract in U2-OS cells but showed significant increase in sensitivity of U2-OS/Pt cells. The phosphoglycoprotein expression was changed by treatment of cisplatin and seed extract of Schisandra chinensis in osteosarcoma rather cisplatin alone [19,20]. In conclusion, Schisandra chinensis seed extract increases the cytotoxic effect of cisplatin through increased apoptosis and anti-proliferative action in osteosarcoma cells.
Conflict of Interest
Authors declare no conflict of interest.
References
- Nikitovic D, Zafiropoulos A, Tzanakakis GN, Karamanos NK, Tsatsakis AM. Effects of glycosaminoglycans on cell proliferation of normal osteoblasts and human osteosarcoma cells depend on their type and fine chemical compositions. Anticancer Res 2005; 25: 2851-2856.
- Perego P, Caserini C, Gatti L, Carenini N, Romanelli S, Supino R, Pezzoni G. A novel trinuclear platinum complex overcomes cisplatin resistance in an osteosarcoma cell system. Mol Pharmacol 1999; 55: 528-534.
- Gonzalez VM, Fuertes MA, Alonso C, Perez JM. Is cisplatin-induced cell death always produced by apoptosis? Mol Pharmacol 2001; 59: 657-663.
- Moalic S, Liagre B, Corbière C, Bianchi A, Dauça M, Bordji K, Beneytout JL. A plant steroid, diosgenin, induces apoptosis, cell cycle arrest and COX activity in osteosarcoma cells. FEBS Lett 2001; 506: 225-230.
- Sunjic SB, Cipak A, Rabuzin F, Wildburger R, Zarkovic N. The influence of 4-hydroxy-2-nonenal on proliferation, differentiation and apoptosis of human osteosarcoma cells. Biofactors 2005; 24: 141-148.
- Martelli L, Di Mario F, Botti P, Ragazzi E, Martelli M, Kelland L. Accumulation, platinum-DNA adduct formation and cytotoxicity of cisplatin, oxaliplatin and satraplatin in sensitive and resistant human osteosarcoma cell lines, characterized by p53 wild-type status. Biochem Pharmacol 2007; 74: 20-27.
- Panossian A, Wikman G. Pharmacology of Schisandra chinensis Bail: an overview of Russian research and uses in medicine. J Ethnopharmacol 2008; 118: 183-212.
- Chang R, Li Y, Yang X, Yue Y, Dou L, Wang Y, Li X. Protective role of deoxyschizandrin and schisantherin A against myocardial ischemia-reperfusion injury in rats. PloS One 2013; 8: e61590.
- Robson H, Meyer S, Shalet SM, Anderson E, Roberts S, Eden OB. Platinum agents in the treatment of osteosarcoma: efficacy of cisplatin vs. carboplatin in human osteosarcoma cell lines. Med Pediatr Oncol 2002; 39: 573-580.
- Wu YJ, Muldoon LL, Neuwelt EA. The chemo-protective agent N-acetylcysteine blocks cisplatin-induced apoptosis through caspase signalling pathway. J Pharmacol Exp Therap 2005; 312: 424-431.
- Seki K, Yoshikawa H, Shiiki K, Hamada Y, Akamatsu N, Tasaka K. Cisplatin (CDDP) specifically induces apoptosis via sequential activation of caspase-8,-3 and-6 in osteosarcoma. Canc chemother Pharmacol 2000; 45: 199-206.
- Komiya S, Gebhardt MC, Mangham DC, Inoue A. Role of glutathione in cisplatin resistance in osteosarcoma cell lines. J Orthop Res 1998; 16: 15-22.
- Shukla SJ, Duan S, Badner JA, Wu X, Dolan ME. Susceptibility loci involved in cisplatin-induced cytotoxicity and apoptosis. Pharmacogen Genom 2008; 18: 253.
- Zhao G, Cai C, Yang T, Qiu X, Liao B, Li W, Ma Q. MicroRNA-221 induces cell survival and cisplatin resistance through PI3K/Akt pathway in human osteosarcoma. PloS One 2013; 8: e53906.
- Simons AL, Ahmad IM, Mattson DM, Dornfeld KJ, Spitz DR. 2-Deoxy-D-glucose combined with cisplatin enhances cytotoxicity via metabolic oxidative stress in human head and neck cancer cells. Cancer Res 2007; 67: 3364-3370.
- Xu Y, Liu Z, Sun J, Pan Q, Sun F, Yan Z, Hu X. Schisandrin B prevents doxorubicin-induced chronic cardiotoxicity and enhances its anticancer activity in vivo. PloS One 2011; 6: e28335.
- Tatewaki N, Nishida H, Yoshida M, Ando H, Kondo S, Sakamaki T, Konishi T. Differential effect of schisandrin B stereoisomers on ATR-mediated DNA damage checkpoint signalling. J Pharmacol Sci 2013; 122: 138-148.
- Liu Z, Zhang B, Liu K, Ding Z, Hu X. Schisandrin B attenuates cancer invasion and metastasis via inhibiting epithelial-mesenchymal transition. PLoS One 2012; 7: e40480.
- Xia YZ, Yang L, Wang ZD, Guo C, Zhang C, Geng YD, Kong LY. Schisandrin A enhances the cytotoxicity of doxorubicin by the inhibition of nuclear factor-kappa B signaling in a doxorubicin-resistant human osteosarcoma cell line. RSC Adv 2015; 5: 13972-13984.
- Liang Y, Zhou Y, Zhang J, Rao T, Zhou L. Pharmacokinetic compatibility of ginsenosides and Schisandra Lignans in Shengmai-san: from the perspective of p-glycoprotein. PLoS One 2014; 9: e98717.