Research Article - Biomedical Research (2019) Volume 30, Issue 1
The methanolic extract of Moringa oleifera attenuates CCl4 induced hepatonephro toxicity in the male rat.
Mohamed A. Elbakry1,2, Haddad A. El Rabey1,3*, Wesam Elremaly1, Mohamed I Sakran1,2 and Fahad M. Almutairi11Biochemistry Department, University of Tabuk, Tabuk, Kingdom of Saudi Arabia
2Section of Biochemistry, Department of Chemistry, Tanta University, Egypt
3Bioinformatics Department, Genetic Engineering and Biotechnology Research Institute, University of Sadat City, Egypt
- *Corresponding Author:
- Haddad A. El Rabey
Biochemistry Department
University of Tabuk, KSA
Accepted on November 24, 2018
DOI: 10.35841/biomedicalresearch.30-18-1056
Visit for more related articles at Biomedical ResearchAbstract
Carbon tetrachloride (CCl4) causes severe injury to the body particularly the liver and the kidney. The aim of the present study was testing the probable hepatonephro protective effect of Moringa oleifera seed methanolic extract against CCl4 toxicity in male rat. 24 male rats were divided into 4 groups (n=6); the first group (G1) was the negative control group, rats of the other three groups were injected with CCl4 twice a week. Rats of the second group (G2) were kept without treatment as positive CCl4 group, the third group (G3) received a daily dose of M. oleifera methanolic extract and the fourth group (G4) received a silymarin dose as a positive treated group. The HPLC analysis of M. oleifera seed methanolic extract revealed that it is rich in ascorbic and oleic acids. Rats of the CCl4 positive control group showed an increase in kidney and liver injury markers, interleukin-6, bilirubin and lipid peroxidation, and a decrease in antioxidants activity and total protein. In addition, liver and kidney tissues showed drastic histopathological changes. Treating the CCl4 hepatonephrotoxicity in G3 and G4 with either M. oleifera seed methanolic extract or silymarin, respectively significantly alleviated all altered biochemical and histological changes approaching the normal values. M. oleifera methanolic extract revealed more protection to liver and kidney in G3 than silymarin in G4. This protecting activity of M. oleifera seed methanolic extract against CCl4 hepatonephrotoxicity may be ascribed to its high content of phenols and flavonoids, in addition to ascorbic acid and oleic acid.
Keywords
Carbon tetrachloride, Moringa oleifera, Hepatotoxicity, Methanol extract, Rat.
List of Abbreviations
A/G: Albumin/Globulin; ALP: Serum Alkaline Phosphatase; ALT: Serum Alanine Aminotransferase; AST: Serum Aspartate Aminotransferase; B.W: Body Weight; BWG%: Body Weight Gain Ratio; BWG: Body Weight Gain; Cat: Catalase; CCl4: Carbon Tetrachloride; Crea: Creatine Kinase-MB; FER: Food Efficiency Ratio; FI: Food Intake; G1: The first group, was untreated control group intraperitoneally injected with 1 ml/kg body weight olive oil; G2: The positive CCl4 control group intraperitoneally injected with CCl4 (1 ml/kg body weight): olive oil (1:1) on the 1st and 4th d of every week for 8 w; G3: The 3rd group, was intraperitoneally injected with CCl4 ((1 ml/kg body weight): olive oil (1:1)) on the 1st and 4th d of every week and cotreated orally with 20% (w/w) Moringa oleifera methanol extract for 8 w using a stomach tube; G4: The 4th group, was intraperitoneally injected with CCl4 ((1 ml/kg body weight): olive oil (1:1)) on the 1st and 4th d of every week and cotreated orally with 0.05 mg/kg body weight silymarin for 8 w; GSST: Glutathione Reductase; GST: Glutathione S-Transferase; H&E: Hematoxylin and Eosin; HPLC: High Performance Liquid Chromatography; IL-6: Interleukin-6; M. oleifera: Moringa oleifera; MDA: Malondialdehyde; ROS: Reactive Oxygen Species; SOD: Superoxide Dismutase; SPSS: Statistical Program for Sociology Scientists; TAC: Total Antioxidant Capacity; UA: Uric Acid.
Introduction
Carbon tetrachloride (CCl4) is commonly used for generating liver [1,2] and kidney injury [3,4] in rat model. CCl4 is metabolized in hepatocytes by the cytochrome P450 to produce free radicals that induce oxidative stress leading to hepatic and renal injury [5]. This induced oxidative stress is a physiological status associated with the unbalance between free radical and antioxidant defenses [1,6].
The induced CCl4 hepatonephrotoxicity also causes increase in blood flow and cytokine accumulation which are characteristic of tissue inflammation [2]. Therefore, protection against CCl4 induced hepatotoxicity is performed through prevention of oxidative damage, or up-regulation of antioxidant genes and down regulation of proinflammatory IL-6 gene which are a possible mechanism of protection [7]. Numerous chemical and natural products were efficiently used in alleviating the toxic effects of CCl4 [8-10].
Moringa oleifera Lamarack (Moringaceae) is known in the developing countries as a vegetable, a medicinal plant, a source of vegetable oil, a forage for livestock, a natural anthelmintic and a boost food security in developing countries [11,12]. The aqueous and alcoholic extract of M. oleifera root-wood reduce the elevated urinary oxalate and lowers the deposition of stone forming constituents of rat kidneys subjected to ethylene glycol treatment [13]. M. oleifera Lam. Also has nutraceutical uses in the treatment of hypercholesterolemia and hyperglycemia [14-16].
In addition, M. oleifera leaf extract succeeded in protecting against liver injury induced by carbon tetrachloride [17,18]. M. oleifera also succeeded in protecting against liver toxicity induced by artesunateamodiaquine antimalarial combination [19]. Moringa also increased wound healing of normal and dexamethasone suppressed wound healing and can be prescribed as food appendage for coronary artery disease patients along with their regular medicines [20,21].
It is worthy to mention that M. oleifera is genotoxic at suprasupplementation levels of 3000 mg/kg body weight [14].
This study aimed to test the protecting activity of the methanolic extract of M. oleifera seed against CCl4 induced hepatonephrotoxicity in the male rat.
Materials and Methods
Carbon tetrachloride and methanol were purchased from Merck Ltd, Coimbatore, Tamil Nadu (India). Silymarin was purchased from a pharmacy in Tabuk, KSA. Virgin olive oil was obtained from a local herbal medicine shop in Tabuk (Saudi Arabia). The seeds of M. oleifera were collected from a farm at Tabouk, identified by a specialized botanist and a specimen was deposited at Biochemistry Department, Faculty of Science, University of Tabouk, Tabouk, Saudi Arabia.
The basal diet
All animals fed standard diet consisted of the following ingredients (obtained from animal feed shop, Tabouk, KSA): 20.0% protein, 4.0% fat, 5.0% fiber, 1.0% vitamin mix, 3.50% mineral mix, 0.25% choline chloride and 66.25% corn starch.
Experiment design
Twenty four rats (Rattus norvegicus) of East Asian origin (weighing 180-200 g) were purchased from Faculty of Pharmacy and all experiments were achieved under approved ethical protocols of the University of Tabuk, Kingdom of Saudi Arabia. The animals were divided 6/cage and kept under observation for two weeks before start of the experiment. The experiment was conducted under controlled condition of lighting (12 h light/12 h dark), temperature (25°C) and humidity (40%). All animals received normal feed and water ad libitum for the 8 weeks. Six rats were chosen randomly as the negative control group and were intraperitonially injected in the 1st and 4th d every week by olive oil (1 ml/kg body weight). The other 18 rats were randomly divided into three groups (G2-G4), and then liver and kidney damage was induced in the 18 rats by injecting the rats in the 1st and 4th day every week by CCl4 (1 ml/kg body weight) [2] by intraperitonial injection with equal amount of olive oil. G2 was the positive CCl4 treated control group. G3 was treated with 200 mg/Kg bw of 20% moringa seed methanolic extract [11] and G4 was treated with 0.05 g/kg b.w. of silymarin (as the recommended drug).
On the last day of the experiment blood was withdrawn from the heart and serum was separated by centrifugation for 15 min at 7000 rpm and 4°C. The animals were rats were anesthetized with diethyl ether, and then sacrificed. The liver and both kidneys were excised immediately and washed with sterile saline solution. One kidney and a piece of liver was washed in saline and fixed in 10% formalin for histopathological section preparations. The other piece of the liver was kept in ice for tissue homogenate preparation.
Preparation of the methanolic extract
The methanolic extract of M. oleifera seeds was prepared according to the method of Adebayo et al. [22]. Briefly, M. oleifera seeds were crushed to powder. 5 g of the sample was soaked in 20 ml of methanol and kept in a rotary shaker for 12 h at 30°C. After that was filtered with Whatmann No.1 filter paper. The filtrate was concentrated in a rotary evaporator until a sticky mass was obtained. The resultant M. oleifera seed methanolic extract was then weighed and stored at 4°C.
HPLC analysis of Moringa seed methanol extract
The methanolic extract of M. oleifera seed was analysed by HPLC using a gradient HPLC (Shimadzu HPLC-20A series, Shimadzu Corpoaration, Japan). Vitamin C (ascorbic acid) was estimated according to the method of Ahmed et al. [23], whereas the fatty acid content was estimated according to the method of Hein et al. [24].
Antioxidants and lipid peroxide assay
Glutathione-s-transferase, catalase, superoxide dismutase, and lipid peroxide were assayed in liver tissue homogenate by Systronics Spectrophotometer, Systronics (India) using Biodiagnostic kit (Egypt), according to the instruction of the manufacturer.
Interleukin-6
The proinflammatory cytokine IL-6 was estimated in liver tissue homogenate by quantitative ELISA analysis using R&D Systems Inc. (United States) according to the instruction of manufacturer.
Markers of liver dysfunction
Serum aspartate aminotransferase (AST) was estimated using Swemed Diagnostics kit (India), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) were estimated using Human kit (Germany) by Systronics Spectrophotometer, Systronics (India) following the manufacturer's instructions.
Kidney function indices
Uric acid and urea were estimated in the serum using Human kit (Germany), whereas creatinine was estimated using Moody International kit (UKAS, Germany) by Systronics Spectrophotometer, Systronics (India).
Serum electrolytes
The levels of Potassium (K+) and sodium (Na+) ions were estimated using Human Kits (Germany) by Systronics Spectrophotometer, Systronics (India).
Estimation of total protein and bilirubin
The total protein and bilirubin were estimated by Systronics Spectrophotometer, Systronics (India). Total protein was estimated using Sigma-Aldrich kit (USA), whereas bilirubin was estimated using Human Kit (Germany).
Histopathology of liver and kidney
Liver and kidney tissues were rinsed in sterile saline and fixed in 10% buffered neutral formalin solution. After fixation, the liver tissues were processed by embedding in paraffin. The paraffin blocks were sectioned and stained with hematoxylin and eosin (H&E). The sections were examined and photographed using Olympus light microscope (Japan).
Physiological evaluation
Body weight gain (BWG, body weight gain percentage, food efficiency and food efficiency ratio were calculated as follows:
Weight gain=Final weight (g)-initial weight (g)
BWG%=Final weight-initial weight/initial weight × 100
FER=Weight (g)/food intake (g)
FER%=Weight (g)/food intake (g) × 100
Statistical analysis
Statistical analyses were carried out using SPSS software to determine the mean and standard error as well as t-test, and then reanalysed by one way ANOVA to determine the best group using SAS package software.
Results
HPLC analysis of M. oleifera methanolic extract
The HPLC analysis (Figure S1) showed that the M. oleifera seed methanolic extract contains 728.2 mg/kg ascorbic acid, whereas the fatty acids content of the M. oleifera seed methanolic extract is shown in Table 1 and Figure S2. Oleic acid was the major constituent (66.8%) and myristic acid was the minor constituent (0.3%).
| Fatty acid | Concentration (%) |
|---|---|
| Oleic acid | 66.8 ± 1.116 |
| Linoleic | 2.58 ± 0.401 |
| Palmitoleic | 7.65 ± 0.654 |
| Arachidic | 7.45 ± 0.643 |
| Behenic | 2.5 ± 0.121 |
| Palmitic | 5.58 ± 0.324 |
| Stearic | 7.26 ± 0.648 |
| Myristic | 0.3 ± 0.106 |
Table 1. Fatty acids content of the HPLC analysed M. oleifera methanol extract.
Antioxidants and lipid peroxidation
Table 2 shows the effect of treating the CCl4 induced hepatotoxic male rats with M. oleifera seed methanolic extract on antioxidants (catalase, super oxide dismutase, glutathione-stransferase and glutathione reduced) and lipid peroxidation (as measured by malondialdehyde “MDA”) in liver tissue homogenate. The antioxidants were significantly reduced, whereas lipid peroxidation was significantly increased in the second positive CCl4 treated group compared to the negative control group (G1). Whereas treating the heptotoxic groups in G3 and G4 with M. oleifera seed methanolic extract and silymarin, respectively significantly increased the antioxidants and decreased lipid peroxidation in the liver tissue homogenate compared to the positive control. Treating the CCl4 induced hepatotoxic rats with M. oleifera seed methanolic extract in G3 was more efficient than treating with silymarin in G4.
| Statistics | G1 | G2 | G3 | G4 | |
|---|---|---|---|---|---|
| Negative control | Positive control | Moringa oleifera | Silymarin | ||
| Catalase (U/g) | Mean ± SE | 4.433 ± 0.181a | 0.616 ± 0.023d | 2.500 ± 0.106b | 1.826 ± 0.022c |
| LSD 0.05=0.341 | |||||
| t-test | - | 21.711*** | -19.386*** | -30.377*** | |
| Superoxide dismutase (U/g) | Mean ± SE | 346.666 ± 18.907a | 158.833 ± 2.271d | 275.333 ± 2.905b | 230.833 ± 3.124c |
| LSD 0.05=31.081 | |||||
| t-test | - | 9.640*** | -39.765*** | -25.246*** | |
| Glutathione reductase (U/g) | Mean ± SE | 317.116 ± 3.634a | 71.316 ± 1.724d | 212.500 ± 4.773b | 165.000 ± 2.756c |
| LSD 0.05=9.050 | |||||
| t-test | - | 55.352*** | -25.722*** | -35.803*** | |
| Glutathione reduced (mmole/g) | Mean ± SE | 315.333 ± 2.616a | 59.333 ± 2.290d | 190.333 ± 5.736b | 131.166 ± 3.166c |
| LSD 0.05=12.517 | |||||
| t-test | - | 71.184*** | -21.673*** | -14.594 | |
| MDA (nmol/g) | Mean ± SE | 1.466 ± 0.098d | 11.900 ± 0.184a | 4.650 ± 0.317c | 7.7167 ± 0.212b |
| LSD 0.05=0.594 | |||||
| t-test | - | -43.405*** | 40.635*** | 15.560*** | |
| Data are represented as mean ± SE. T-test values: ***significant at P<0.001. ANOVA analysis: within each row, means with different superscript (a-c or d) are significantly different at P<0.05, whereas means superscripts with the same letters mean that there is no significant difference at P>0.05. LSD: Least Significant Difference. | |||||
Table 2. Effect of M. oleifera methanol extract supplementation for 8 w on antioxidants and lipid peroxidation in tissue homogenate of CCl4 hepatonephrotoxic male rat.
Interleukin-6
Table 3 shows that the interleukin-6 (IL-6) in liver tissue homogenate of CCl4 hepatootoxic male rats was increased in the tissue homogenate of the CCl4 positive control group (G2). Treating the CCl4 administered groups with M. oleifera and silymarin in the third and the fourth group, respectively decreased the IL-6 levels compared to the positive control group. Similar to other results M. oleifera seed methanolic extract was more efficient than silymarin.
| Parameters | Statistics | G1 | G2 | G3 | G4 |
|---|---|---|---|---|---|
| N. control | P. control | Moringa oleifera | Silymarin | ||
| IL6 (pg/g.tissue) | Mean ± SE | 44.583 ± 1.467d | 88.866± 1 .312a | 71.100 ± 1.049c | 78.400 ± 0.972b |
| LSD 0.05=3.647 | |||||
| T-test | - | -33.954*** | 10.893*** | 6.147*** | |
| Data are represented as mean ± SE. t-test values: ***significant at P<0.001. ANOVA analysis: within each row, means with different superscript (a-c or d) are significantly different at P<0.05, whereas means superscripts with the same letters mean that there is no significant difference at P>0.05. LSD: Least Significant Difference. | |||||
Table 3. Effect of M. oleifera methanol extract supplementation on interleukin-6 in tissue homogenate of CCl4 hepatonephrotoxic male rats.
Liver enzymes
Table 4 shows the effect of treating CCl4 induced hepatotoxic male rats with M. oleifera methanol extract on liver enzymes. The second positive CCl4 treated group showed elevated liver enzymes compared to the negative control group. Treating the heptotoxic groups in G3 and G4 with M. oleifera seed methanolic extract and silymarin, respectively significantly reduced the liver enzymes activity compared to the positive control group but still higher than the negative control. M. oleifera methanol extract was more efficient than silymarin.
| Liver enzymes | Groups | G1 | G2 | G3 | G4 |
|---|---|---|---|---|---|
| N. control | P. control | Moringa oleifera | Silymarin | ||
| Statistics | |||||
| ALT (U/l) | Mean ± SE | 21.666 ± 1.452d | 98.500 ± 2.140a | 71.333 ± 1.333c | 84.666 ± 1.282b |
| LSD 0.05=4.699 | |||||
| t-test | - | -28.192*** | 28.725*** | 5.099*** | |
| AST (U/l) | Mean ± SE | 22.333 ± 1.145d | 91.833 ± 2.242a | 66.333 ± 1.819c | 76.333 ± 0.881b |
| LSD 0.05=5.355 | |||||
| t-test | - | -47.035*** | 7.286*** | 5.654*** | |
| ALP (U/l) | Mean ± SE | 164.000 ± 3.521b | 283.333 ± 2.836a | 220.166 ± 3.970a | 254.333 ± 2.185a |
| LSD 0.05=37.033 | |||||
| t-test | - | -33.041*** | 11.014*** | 6.618*** | |
| Data are represented as mean ± SE. t-test values: ***significant at P<0.001. ANOVA analysis: within each row, means with different superscript (a-c or d) are significantly different at P<0.05, whereas means superscripts with the same letters mean that there is no significant difference at P>0.05. LSD: Least Significant Difference. | |||||
Table 4. Effect of M. oleifera methanol extract supplementation for 8 w on liver enzymes (ALT, AST and ALP) in CCl4 hepatonephrotoxic male rat.
Renal indices
Table 5 shows that the levels of blood urea nitrogen, creatinine and uric acid were significantly elevated in the second positive CCl4 treated group compared to the negative control group. While treating the CCl4 administered groups in G3 and G4 with M. oleifera seed methanolic extract and silymarin, respectively significantly reduced these kidney functions parameters compared to the positive control group approaching the negative control. Moreover, sodium and potassium ions were significantly reduced with CCl4 hepatonephrotoxicity in G2 and were elevated with M. oleifera seed methanolic extract and silymarin treatment in G3 and G4, respectively. Similar to the liver function parameters, M. oleifera seed methanolic extract was more efficient than silymarin in treating the altered kidney function parameters.
| Parameters | Statistics | G1 | G2 | G3 | G4 |
|---|---|---|---|---|---|
| Negative control | Positive control | Moringa oleifera | Silymarin | ||
|  Blood urea nitrogen (BUN) mg/dl | Mean ± SE | 22.000 ± 1.064d | 64.000 ± 1.316a | 48.833 ± 1.301c | 56.833 ± 0.792b |
| LSD 0.05=3.662 | |||||
| t-test | - | -23.238*** | 6.013*** | 4.366*** | |
| Creatinine (mg/dl) | Mean ± SE | 0.718 ± 0.026d | 2.516 ± 0.116a | 1.266 ± 0.066c | 1.900 ± 0.057b |
| LSD 0.05=0.220 | |||||
| t-test | - | -18.904*** | 7.986*** | 4.561*** | |
| Uric acid (mg/dl) | Mean ± SE | 4.250 ± 0.076d | 7.283 ± 0.119a | 6.250 ± 0.076c | 6.866 ± 0.042b |
| LSD 0.05=0.271 | |||||
| t-test | - | -20.555*** | 6.609*** | 3.027*** | |
| Sodium (Na+) | Mean ± SE | 146.000 ± 0.930a | 117.166 ± 1.166c | 127.166 ± 0.792b | 119.833 ± 0.703d |
| LSD 0.05=2.729 | |||||
| t-test | - | 23.586*** | -6.956*** | -2.123* | |
| Potassium (K+) | Mean ± SE | 100.000 ± 1.527a | 71.500 ± 1.995c | 90.333 ± 0.881b | 97.500 ± 0.991d |
| LSD 0.05=4.356 | |||||
| t-test | - | 14.783*** | -8.455*** | -10.331*** | |
| Data are represented as mean ± SE. t-test values: ***significant at P<0.001. ANOVA analysis: within each row, means with different superscript (a-c or d) are significantly different at P<0.05, whereas means superscripts with the same letters mean that there is no significant difference at P>0.05. LSD: Least Significant Difference. | |||||
Table 5. Effect of M. oleifera methanol extract supplementation for 8 w on kidney function and electrolytes in the serum of CCl4 hepatonephrotoxic male rats.
Serum proteins and bilirubin
Table 6 shows that the total protein level was significantly reduced, whereas bilirubin was significantly increased in the second positive CCl4 treated group compared to the negative control group. While treating the heptonephrotoxic groups in G3 and G4 with M. oleifera seed methanolic extract and silymarin, respectively significantly increased the total protein and reduced bilirubin compared to the positive control (G2). Treating the CCl4 induced hepatotoxic rats with M. oleifera seed methanolic extract in G3 was more efficient than treating with silymarin in G4.
| Statistics | G1 | G2 | G3 | G4 | |
|---|---|---|---|---|---|
| Negative control | Positive control | Moringa oleifera | Silymarin | ||
| Total protein (g/dl) | Mean ± SE | 5.933 ± 0.114a | 4.316 ± 0.083d | 4.900 ± 0.106b | 4.866 ± 0.061c |
| LSD 0.05=0.241 | |||||
| t-test | - | 1.149*** | -1.566*** | -1.975*** | |
| Albumin (g/dl) | Mean ± SE | 3.500 ± 0.057a | 2.316 ± 0.090d | 3.000 ± 0.116b | 3.206 ± 0.066c |
| LSD 0.05=0.199 | |||||
| t-test | - | 2.053*** | -1.149*** | -1.551*** | |
| Globulin (g/dl) | Mean ± SE | 2.50 ± 0.125c | 2.000 ± 0.085a | 1.910 ± 0.132b | 1.700 ± 0.115d |
| LSD 0.05=0.269 | |||||
| t-test | - | -1.139*** | 1.452*** | 0.000NS | |
| A/G ratio | Mean ± SE | 1.4 ± 0.890a | 1.158 ± 0.086b | 1. 57 ± 0.278c | 1.29 ± 0.105d |
| LSD 0.05=1.37 | |||||
| t-test | - | 4.994*** | -6.346*** | -6.263*** | |
| Total bilirubin (mg/dl) | Mean ± SE | 0.428 ± 0.014d | 1.516 ± 0.079a | 0.725 ± 0.012c | 0.895 ± 0.019b |
| LSD 0.05=0.132 | |||||
| t-test | - | -1.274*** | 1.460*** | 1.356*** | |
| Data are represented as mean ± SE. t-test values: ***significant at P<0.001. ANOVA analysis: within each row, means with different superscript (a-c or d) are significantly different at P<0.05, whereas means superscripts with the same letters mean that there is no significant difference at P>0.05. LSD: Least Significant Difference. | |||||
Table 6. Effect of M. oleifera methanol extract supplementation for 8 w on serum proteins and bilirubin in CCl4 hepatonephrotoxic male rat.
Physiological evaluation
Table 7 shows that body weight gain (per day and per week), percentage of body weight gain, food efficiency ratio and percent of food efficiency ratio were decreased as a result of hepatotoxicity induction using CCl4 in the second group compared to the negative control group, whereas these values were increased approaching the normal value as in G1 with the cosupplementation with M. oleifera seed methanolic extract in G3, and silymarin in G4.
| Physiological evaluation | Statistics | G1 | G2 | G3 | G4 |
|---|---|---|---|---|---|
| Negative control | Positive control | Moringa oleifera | Silymarin | ||
| BWG (g/d) | Mean ± SE | 1.238 ± 0.045a | -0.877 ± 0.018d | 1.033 ± 0.028b | 0.972 ± 0.020c |
| LSD 0.05=3.031 | |||||
| t-test | 3.315*** | -5.067*** | -6.412*** | ||
| BWG (g/8 w) | Mean ± SE | 37.166 ± 1.351a | -26.333 ± 0.557d | 31.000 ± 0.856b | 27.166 ± 0.600c |
| LSD 0.05=1.632 | |||||
| t-test | - | 3.360*** | -5.100*** | -6.518*** | |
| BWG (%) | Mean ± SE | 20.645 ± 0.737a | -14.233 ± 0.287d | 16.317 ± 0.463b | 15.584 ± 0.307c |
| LSD 0.05=0.101 | |||||
| t-test | - | 3.285*** | -5.924*** | -6.954*** | |
| FER (g/d) | Mean ± SE | 0.0723 ± 0.002a | -0.0513 ± 0.000d | 0.060 ± 0.001b | 0.054 ± 0.001c |
| LSD 0.05=0.005 | |||||
| t-test | - | 3.771*** | -5.281*** | -6.078*** | |
| FER (%) | Mean ± SE | 7.256 ± 0.263a | 5.124 ± 0.108d | 6.006 ± 0.165b | 5.392 ± 0.118c |
| LSD 0.05=0.591 | |||||
| t-test | - | 36.340*** | -56.126*** | -60.387*** | |
| Data are represented as mean ± SE. t-test values: ***significant at P<0.001. ANOVA analysis: within each row, means with different superscript (a-c or d) are significantly different at P<0.05, whereas means superscripts with the same letters mean that there is no significant difference at P>0.05. LSD: Least Significant Difference. | |||||
Table 7. Effect of M. oleifera methanol extract supplementation for 8 w on physiological evaluation in CCl4 hepatonephrotoxic male rat.
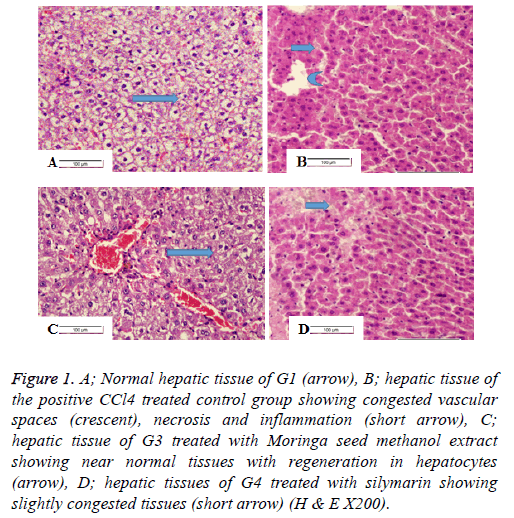
Histopathology of liver
Figure 1A shows the liver of rat from group 1 showing the normal hepatic tissues of hepatic lobule. Figure 1B shows liver of rat from group 2 showing congestion in vascular spaces, necrosis and marked inflammation. Figure 1C showing liver of rat from group 3 treated with Moringa seed methanolic extract showing restored near normal hepatic tissues with regeneration in hepatocytes. Figure 1D shows the hepatic tissue of rat from the 4th group treated with silymarin with slight inflammation and nearly normal hepatic tissues.
Figure 1: A; Normal hepatic tissue of G1 (arrow), B; hepatic tissue of the positive CCl4 treated control group showing congested vascular spaces (crescent), necrosis and inflammation (short arrow), C; hepatic tissue of G3 treated with Moringa seed methanol extract showing near normal tissues with regeneration in hepatocytes (arrow), D; hepatic tissues of G4 treated with silymarin showing slightly congested tissues (short arrow) (H & E X200).
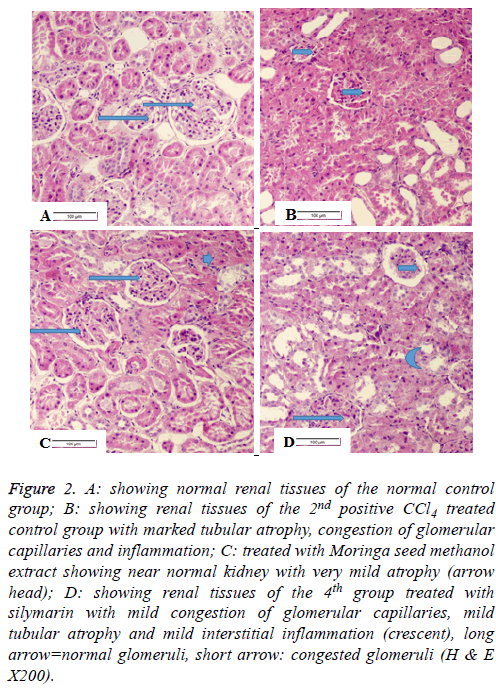
Histopathology of kidney
Renal tissues of the negative control group (G1) showed normal renal tissues (Figure 2A) with normal histological structure of renal parenchyma, blood vessels, normal glomeruli, normal renal tubuli and normal interstitial tissue with normal blood vessels. Figure 2B shows renal tissues of the 2nd positive control group with marked tubular atrophy, congestion of glomerular capillaries and moderate inflammation. Figure 2C shows nearly normal renal tissues of the 3rd group treated with moringa seed methanolic extract with nearly normal very mild atrophy. Figure 2D shows renal tissues of the 4th group treated with silymarin with mild congestion of glomerular capillaries, mild tubular atrophy with mild interstitial inflammation.
Figure 2: A: showing normal renal tissues of the normal control group; B: showing renal tissues of the 2nd positive CCl4 treated control group with marked tubular atrophy, congestion of glomerular capillaries and inflammation; C: treated with Moringa seed methanol extract showing near normal kidney with very mild atrophy (arrow head); D: showing renal tissues of the 4th group treated with silymarin with mild congestion of glomerular capillaries, mild tubular atrophy and mild interstitial inflammation (crescent), long arrow=normal glomeruli, short arrow: congested glomeruli (H & E X200).
Discussion
The aim of the present study was to study the hepatonephro protective activity of M. oleifera seed methanol extract against CCl4 toxicity in male rats. Silymarin was used in protecting against CCl4 hepatotoxicity in the fourth group as the recommended drug worldwide to evaluate the protecting activity of M. oleifera seed methanolic extract. The HPLC analysis of the methanolic extract of the tested M. oleifera seed contained a higher content of the antioxidants; oleic acid (which is a monounsaturated omega-9 fatty acid) and ascorbic acid (which is s a major antioxidant and plays an important role in plant growth and development), in addition to the other known phenolic and flavonoid compounds [25].
CCl4 toxicity decreased all antioxidants and increased the oxidative stress as revealed by the increased lipid peroxidation in the positive control group [2,4]. However, M. oleifera seed methanolic extract succeeded in increasing the antioxidant enzyme activity and decreasing the oxidative stress by decreasing lipid peroxidation. Oleic acid and ascorbic acid were the major constituent of the tested sample of M. oleifera seed methanolic extract compared to the other constituents. This protecting activity of M. oleifera against CCl4 toxicity is consistent with previous investigations revealed that the crude extract of M. oleifera is a good scavenger for nitric oxide radicals and has a potential source of natural antioxidant [16,26,27]. In addition, oleic acid reduces blood pressure by increasing fat burning and protects cells from free radical damage [28]. Whereas ascorbic acid (vitamin C) in addition to its immunological benefits it was recently found that it protects against endothelial dysfunction, carcinogenicity, hyperglycemia, cardiac health, degenerative diseases and the blood vessel changes that precede heart disease [29-31].
Similarly, interleukin-6 level was also increased as an indication of inflammatory effect resulted from CCl4 toxicity due to the up regulation of IL-6 gene [2]. The antioxidant activity of M. oleifera seed methanolic extract succeeded in alleviating this inflammatory effect as revealed by the decreased Il-6 levels in the third group. Down regulation of the proinflammatory IL-6 gene is a possible mechanism of protection against CCl4 toxicity [7]. It is also worthy to mention that all studied parameters showed that treating the CCl4 administered rats with M. oleifera seed methanolic extract is much more efficient than silymarin which was used in this study as the recommended drug for treating liver cirrhosis worldwide.
CCl4 toxicity greatly injured both liver and kidney as revealed by the elevated liver enzyme activity and kidney function parameters, and the decreased serum sodium and potassium ions in the positive control group [1-4]. Moreover, CCl4- induced hepatotoxicity profound elevation of reactive oxygen species (ROS) production and oxidative stress, as revealed by the increase of lipid peroxidation level and depleting of the total antioxidant capacity (TAC) in liver [2,8]. Since uric acid and urea resulted from nucleic acid and protein catabolism, respectively, the elevation in uric acid is ascribed to the increase in protein catabolism, whereas the enhanced amino acid deamination for gluconeogenesis may cause elevation in the levels of urea [2].
The M. oleifera seed methanolic extract has an ameliorative effect on liver fibrosis in rats by reducing liver damage and symptoms of liver fibrosis, decreasing the CCl4-induced elevation of serum aminotransferase activities and globulin level, and reducing the elevated hepatic hydroxyproline content and myeloperoxidase activity [17,26].
On the other hand, treating the CCl4 injured liver and kidney with M. oleifera seed methanolic extract and silymarin attenuated these toxic effects and decreased the elevated liver and kidney function parameters approaching the normal levels. This is might be ascribed to the antioxidant activity of M. oleifera seed methanolic extract. In addition, other herbs such as Ginseng essence could ameliorate liver fibrosis via inhibiting the activation of hepatic stellate cells through elevating the activities of antioxidative enzymes and the content of antioxidant [32]. The significant decrease in serum total protein as a result of CC14 toxicity in the second positive control group was due to the reduction in forming hepatic protein [2,33].
The decrease in BWG% and FER as a result of CCl4 toxicity in the second group compared with negative control group is consistent with Fang et al. [34] and Al-Seeni et al. [2]. Similar to other studied parameters, the value of BWG and FER was significantly increased with M. oleifera seed methanolic extract and silymarin in the 3rd and 4th group, respectively due to the antioxidant and alleviating activity of M. oleifera seed methanol extract.
Concerning limitation and further studies, most literature recommended the silymarin dose to be 0.05 mg/kg bw [35,36]. In future studies, we recommend the increase of that dose to be 0.07 mg/kg bw. In addition, the dose of M. oleifera seed methanol extract must be increased to give better results (for example: 250-300 mg/kg bw of 20% seed methanol extract). Furthermore, the bioavailability of different formulations of Moringa oleifera seed methanol extract should be taken into account in the future studies, too.
The current study showed that HPLC analysis of the methanolic extract of M. oleifera seeds contains higher percentage of the antioxidants; oleic acid, ascorbic acid, in addition to phenols and flavonoid. These constituents are highly antioxidants and succeeded in protecting-to a higher extent-against the toxic effect of carbon tetrachloride on liver and kidney by alleviating these effects on both biochemical and histopathological levels approaching the negative control condition. It is also worthy to mention that the methanolic extract of M. oleifera seeds was more efficient in protecting both liver and kidney against CCl4 toxicity than silymarin.
Conflict of Interest
The authors declared no conflict of interests
Acknowledgements
The project was funded by the Deanship of Scientific Research (DSR), University of Tabuk, Tabuk, KSA under grant No. (S-1438-0088). The authors, therefore, acknowledge with thanks DSR technical and financial support.
References
- Minami K, Saito T, Narahara M. Relationship between hepatic gene expression profiles and hepatotoxicity in five typical hepatotoxicant administered rats. Toxicol Sci 2005; 87: 296-305.
- Al-Seeni MN, El Rabey HA, Zamzami MA. The hepatoprotective activity of olive oil and Nigella sativa oil against CCl4 induced hepatotoxicity in male rats. BMC Compl Alt Med 2016; 16: 438.
- Rincón AR, Covarrubias A, Pedraza-Chaverrí J, Poo JL, Armendáriz-Borunda J, Panduro A. Differential effect of CCl4 on renal function in cirrhotic and non-cirrhotic rats. Exp Toxicol Pathol 1999; 51: 199-205.
- Jan S, Khan MR. Protective effects of Monotheca buxifolia fruit on renal toxicity induced by CCl4 in rats. BMC Compl Alt Med 2016; 16: 289.
- Yang C, Gong X, Ai Q. 5-aminoimidazole-4-carboxamide-1-B-Dribofuranoside alleviated carbon tetrachloride-induced acute hepatitis in mice. Int. Immunopharmacol 2015; 25: 393-399.
- Moselhy S, Ali H. Hepatoprotective effect of cinnamon extracts against carbon tetrachloride induced oxidative stress and liver injury in rats. Biol Res 2009; 42: 93-98.
- Bhondave PD, Devarshi PP, Mahadik KR. Ashvagandharishta prepared using yeast consortium from Woodfordia fruticosa flowers exhibit hepatoprotective effect on CCl4 induced liver damage in Wistar rats. J Ethnopharm 2014; 151: 183-190.
- Ma J, Ding J, Zhang L, Liu C. Hepatoprotective properties of sesamin against CCl4 induced oxidative stress-mediated apoptosis in mice via JNK pathway. Food Chem Toxicol 2014; 64: 41-48.
- Tsai J, Chiu C, Chen Y. Hepatoprotective effect of Coreopsis tinctoria flowers against carbon tetrachloride-induced liver damage in mice. BMC Compl Alt Med 2017; 17: 139.
- Gupta R, Dubey DK, Kannan GM. Concomitant administration of Moringa oleifera seed powder in the remediation of arsenic-induced oxidative stress in mouse. Cell Bio Int 2007; 31: 44-56.
- Mahajan SG, Mali RG, Mehta AA. Protective effect of ethanolic extract of seeds of Moringa oleifera Lam. against inflammation associated with development of arthritis in rats. J Immunotoxicol 2007; 4: 39-47.
- Karadi RV, Gadge NB, Alagawadi KR, Savadi RV. Effect of Moringa oleifera Lam. root-wood on ethylene glycol induced urolithiasis in rats. J Ethnopharmacol 2006; 105: 306-311.
- Asare GA, Gyan B, Bugyei K. Toxicity potentials of the nutraceutical Moringa oleifera at supra supplementation levels. J Ethnopharm 2012; 139: 265-272.
- Al-Malki AL, El Rabey HA. The antidiabetic effect of low doses of Moringa oleifera Lam. seeds on streptozotocin induced diabetes and diabetic nephropathy in male rats. BioMed Res Int 2015; 381040.
- El Rabey HA, Khan JA, Almutairi FM. The low dose of drumsticks (Moringa oleifera L.) seed powder ameliorates blood cholesterol in hypercholesterolemic male rat. Indian J Biochem Biophys 2017; 54: 306-313.
- Ujah OF, Ujah IR, Johnson JT. Hepatoprotective property of ethanolic leaf extract of Moringa oleifera on carbon tetrachloride (CCl4) induced hepatotoxicity. J Nat Prod Plant Resour 2013; 3: 15-22.
- El-Bakry K, Toson E, Serag M. Hepatoprotective effect of Moringa oleifera leaves extract against carbon tetrachloride- induced liver damage in rats. World J Pharm Pharm Sci 2016; 5: 76-89.
- Okumu MO, Ochola FO, Mbaria JM. Mitigative effects of Moringa oleifera against liver injury induced by artesunateamodiaquine antimalarial combination in Wistar rats. Clin Phytosci 2017; 3: 18.
- Vijay L, Kumar U. Effect of Moringa oleifera Lam. on normal and dexamethasone suppressed wound healing. Asian Pac J Trop Biomed 2012; 2: 219-223.
- Rajanandh MG, Satishkumar MN, Elango K. Moringa oleifera Lam. A herbal medicine for hyperlipidemia: a preclinical report. Asian Pac J Trop Dis 2012; 2: 790-795.
- Adebayo E, Ishola O, Taiwo O. Evaluations of the methanol extract of Ficus exasperata stem bark, leaf and root for phytochemical analysis and antimicrobial. Afr J Plant Sci 2009; 3: 283-287.
- Ahmed KS, Banik R, Hossain MH. Vitamin C (L-ascorbic acid) content in different parts of Moringa oleifera grown in Bangladesh. Amer Chem Sci J 2016; 1-6.
- Hein M, Isengard H. Determination of underivated fatty acids by HPLC. Lebensm Unters Forsch 1997; 204: 420-424.
- Sankhalkar S, Vernekar V. Quantitative and qualitative analysis of phenolic and flavonoid content in Moringa oleifera Lam and Ocimum tenuiflorum L. Pharmacogn Res 2016; 8: 16-21.
- Hamza AA. Ameliorative effects of Moringa oleifera Lam seed extract on liver fibrosis in rats. Food Chem Toxicol 2010; 48: 345-355.
- Kumbhare MR, Guleha V, Sivakumar T. Estimation of total phenolic content, cytotoxicity and in-vitro antioxidant activity of stem bark of Moringa oleifera. Asian Pac J Trop Dis 2012; 2: 144-150.
- de Silva PS, Luben R, Shrestha SS. Dietary arachidonic and oleic acid intake in ulcerative colitis etiology: a prospective cohort study using 7-day food diaries. Eur J Gastroenterol Hepatol 2014; 26: 11-18.
- Rossig L, Hoffmann J, Hugel B, Mallat Z, Haase A, Freyssinet JM, Tedgui A, Aicher A, Zeiher AM, Dimmeler S. Vitamin C inhibits endothelial cell apoptosis in congestive heart failure. Circulation 2001; 104: 2182-2187.
- Salonen RM, Nyyssonen K, Kaikkonen J. Six-year effect of combined vitamin C and E supplementation on atherosclerotic progression: the Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) Study. Circulation 2003; 107: 947-953.
- Guz J, Dziaman T, Szpila A. Do antioxidant vitamins influence carcinogenesis?. Postepy Hig Med Dosw 2007; 61: 185-198.
- Weng C, Lu K, Sheen L. Protective effects of ginseng essence on CCl4-induced oxidative stress and liver injury in rats. Abstracts Eur J Integr Med 2014; 6: 125-132.
- Salem M, El-Rasheid H, Mahmoud A. Therapeutic effects of curcumin and royal jelly as natural antioxidants on some biochemical parameters in hepatotoxicity induced by carbon tetrachloride (CCl4) in male albino rats. Int J Adv Res 2015; 3: 520-535.
- Fang HL, Lai JT, Lin WC. Inhibitory effect of olive oil on fibrosis induced by carbon tetrachloride in rat liver. Clin Nutr 2008; 27: 900-907.
- Imamoto R, Okano J, Sawada S. Null anticarcinogenic effect of silymarin on diethylnitrosamine-induced hepatocarcinogenesis in rats. Exp Ther Med 2014; 7: 31-38.
- Girish C, Pradhan SC. Hepatoprotective activities of picroliv, curcumin, and ellagic acid compared to silymarin on carbon-tetrachloride-induced liver toxicity in mice. J Pharmacol Pharmacother 2012; 3: 149-155.